Introduction
Earthworms, terrestrial oligochaetes (Annelida, Clitelata), have long been recognized as soil benefactors and their presence is commonly associated with good quality soils1. Recently, a comprehensive review of the effects of earthworms on soil highlights their role in catalyzing ecosystem support services such as soil formation and nutrient cycling2. Several studies have proven how oligochaetes favor soil, by improving its physical properties (structure, porosity, bulk density), soil water properties (water regulation, infiltration and runoff), chemical properties (accelerating N mineralization), and biological properties (influencing the structure of the microbial community, resulting in some cases in biological control of diseases and pests)2)(3)(4.
Although earthworms are sometimes referred to as a homogeneous group, more than 5000 different species are recognized in the world5. They vary in size, from barely some centimeters to several meters long, and in behaviour determining a particular depth of residence in the soil, and levels of incidence on surface or in drilosphere (part of the soil influenced by earthworm secretions6). A comprehensive understanding of local earthworm biodiversity enables the prediction of the potential ecosystem services they could offer2, as well as the identification of potential threats caused by the introduction of exotic species7.
Historically, earthworm studies in Uruguay have been scarce. The first oligochaete researcher in Uruguay, born at the end of the 19th century, was Professor Ergasto Cordero, who described several native species. He contributed to systematics, taxonomy and biogeography, in particular to the Glossoscolecidae family, establishing their distribution and phylogeny8)(9)(10. Unfortunately, as he had no followers, earthworm studies in Uruguay were interrupted, and resumed more than half a century later by Grosso and others11 and Grosso & Brown12, Zerbino13)(14)(15, and Zerbino and others16. It is difficult to determine if native species have been displaced, since there has been no systematic study of native earthworms in natural ecosystems to use as a baseline11, i.e. actual local earthworm richness (past and present) is not yet known. So far, 19 species of earthworms have been reported in Uruguay (not all have been identified to the species level), of which more than half are exotic species12. Most probably, with a greater sampling effort, this species list could be expanded, particularly within the natives group, since the majority of the studies have been carried out in agroecosystems, where exotics are more competitive11)(13)(14)(15)(16.
Ten out of 11 exotic species found in Montevideo belong to the Lumbricidae family, Eurasian origin, and the remaining species of the genus Amynthas, belong to the Megascolecidae. Only two native species have been recorded for this province: Microscolex dubius Fletcher, 1887 and Eukerria stagnalis Beddars, 189512. In northwest surveys, only native Oligochaeta, belonging to the families Ocnerodrilidae and Glossoscolecidae, have been collected17.
Morphological differences in earthworms have so far been the only elements to discern species. For instance, Sims and Gerard18 prepared an identification key for British Lumbricids based solely on external characters such as setae arrangement, genital pores shape and position, clitellum position and length, conspicuous genital marks position and shape. However, most external features are only observable in mature individuals and are often not enough to distinguish between species, particularly in the case of non-European species19. Hence, the number, position and shape of internal organs have been used to complete species identification19)(20. Dichotomous keys facilitate identification, assuming that the universe of earthworms for the sampled area is already known and comprehended by the key. Consequently, these keys are site-specific, and cannot be used adequately in a different area from which they were made for.
Currently, species identification by morphology can be complemented by molecular techniques, especially when inter-specific differences depend on internal characters or are distinguishable only in sexually mature individuals. In particular, the use of «DNA barcoding» based on a standardized region of the mitochondrial cytochrome oxidase I gene (COI) has been widely used as a genetic marker to discriminate animal species21. This methodology allowed the successful identification of earthworm species in Asia, Europe and America22)(23)(24. However, when establishing phylogenetic relationships, it is not conclusive, and it is advisable to use more than one genetic marker23)(25)(26. No previous study has investigated molecular methods for earthworm identification in Uruguay.
This study aimed to introduce the use of molecular techniques for identification of earthworm species in Uruguay. It was based on an earthworm collection from samplings in different agricultural soils in Montevideo and Paysandú, Uruguay. To assess the applicability of molecular methods in Uruguay, the samplings covered a range of managements, which included several cropping systems, tillage or no-till sowing and organic or non-organic production, in order to achieve variability and obtain a comprehensive range of species for COI sequencing.
Material and Methods
Sampling sites
Field data were obtained from Typic Argiudolls (according to USDA soil taxonomy) of two provinces in western (Paysandú, 32.5ºS; 58ºW) and southern (Montevideo, 34.5ºS; 56ºW) Uruguay. In Paysandú, the sampling sites were long-term trial plots at the University Experiment Field, with different crop rotations (continuous crops and crop-pasture rotations). In Montevideo, the sampling sites were organic transition farms with tillage and no use of synthesized agrochemicals; and non-organic farms with no-till and use of synthesized fertilizers, herbicides and pesticides.
Sample management and morphological classification
Samplings were conducted in autumn and spring of 2014 and in spring 2015 with precipitations above the average (> 100 mm per month); no sampling was held during autumn 2015 due to a severe drought, since it was not expected to find earthworms in the first 20 cm of soil11. A similar method to that recommended by Anderson and Ingram27 was applied, with five sample units (soil monolith 25 cm x 25 cm and 20 cm depth) per site. In the laboratory, earthworms were hand sorted from monoliths, rinsed with distilled water and gently dried with paper napkins. Earthworms were then anesthetized gradually in alcohol until reaching a concentration of 20 % for later fixation with 4 % formaldehyde solution19.
A total of 1636 earthworms were analyzed, 26 % of which were adults. All adult specimens were grouped by morphology and identified at species level when possible. Classification as natives or exotics was based on the worm list described for Uruguay by Grosso and Brown12, and following the available keys and taxonomic descriptions18)(28)(29)(30. In the absence of a specific key for Uruguay, the dichotomous options of keys were not strictly followed, and where rather used as a guide. For each site, one specimen per morphologic group was molecularly analyzed. For each morphological group, DNA was extracted from the rear section of representative earthworms, which was not fixed in formaldehyde, but preserved in anhydrous ethanol at -20 oC.
Molecular techniques and DNA sequence processing
DNA extraction and polymerase chain reaction (PCR) amplification of the mitochondrial cytochrome oxidase I complex (COI) gene region was performed in LaTraMA laboratory, Biochemistry Faculty, Science College, Universidad de la República, Uruguay. Total DNA was extracted from tissue samples, following a protocol of regular use in LaTraMA, which is a modification of Dellaporta and others31, where an additional chloroform:isoamylic alcohol (24:1) step was included to remove protein excess. Partial mitochondrial COI region was amplified with primers LCO1490 and HCO219832, applying a standard barcoding protocol33 with minor modifications. PCR amplifications were performed in an Axygen™ MaxyGene™ Gradient Thermal Cycler (Axygen Scientific THERM1001, USA) subjected to: 3 min of denaturation at 94 oC, followed by 38 cycles at 94 ºC for 30 s, annealing at 52 ºC for 45 s and 1 min at 72 ºC, followed by a final elongation step of 10 min at 72 ºC, and hold at 20 ºC.
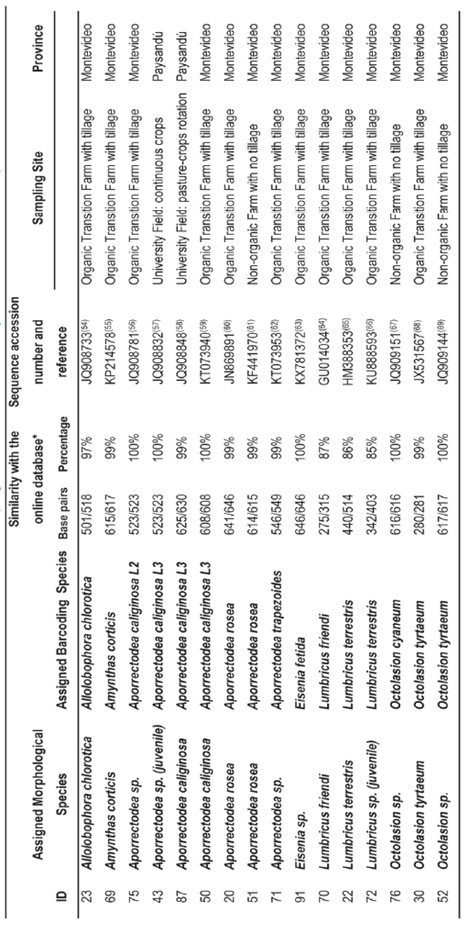
The PCR mix contained 2.5 uL of PCR buffer 10x, 1.25 uL MgCl2 50 mM, 0.25 uL dNTP 2 mM, 0.25 uL Taq 5U/uL, 1.25 uL of each primer, and 2 uL of DNA solution. A final volume of 25 uL was obtained by addition of mQ water. The PCR products were sent to Macrogen (Korea) for purification and sequencing. The DNA sequences were edited manually with Chromas Lite software34 and subjected to Nucleotide Basic Local Alignment Search Tool (BLAST-N35) in NCBI GenBank36. The best matching species, published in indexed journals, was recorded for each query and included in Table 1. Sample sequences of the same species was checked for similarity with Molecular Evolutionary Genetics Analysis (MEGA) software37, when similarity reached 100 % only one specimen per species was included in Table 1.
Results and Discussion
A total of 16 earthworm species, four native and 12 exotic, were identified by morphological taxonomy classification. Three of them have apparently not been previously reported in Uruguay and need deeper analysis for an accurate taxonomical classification. Only one of the native species had the barcoding COI sequence annotated in the database, namely Microscolex dubius, Fletcher, 1887, with two sequences deposited38. This is a low number of accessions considering that Aporrectodea caliginosa, Savigny 1826, for example, has 357 COI sequences in GenBank, several of which are published in indexed journals. Natives were therefore not further analysed in the present study.
There is a reference database, RefSeq39 with cured sequences, i.e. peer reviewed, in which well annotated and not redundant sequences are guaranteed. Although RefSeq39 has more than 55,000 organism sequences, up to date (verified 2018, August 27), only two earthworm COI sequences are reported there: complete mitochondrial genomes for Amynthas jiriensis and Lumbricus terrestris40. Therefore, RefSeq39 has still not become a reference database for terrestrial oligochaete studies.
The COI fragment of one of the unreported species failed to amplify accurately, therefore, more samples of this species should be collected to repeat DNA extraction and amplification. Eight out of 11 successfully sequenced exotic species (35 specimens) were fully identified and matched morphological characters and molecular information; two were less consistent, with lower similarity percentage; and one (two specimens) could not be fully identified due to the lack of close related sequences in GenBank. The finding of most exotic earthworm COI sequences in the available online database, the GenBank reference repository41, is most probably because these belong to the Lumbricidae (originally Holarctic) and Megascolecidae (Asian) families. These families ranked as the first and second «most abundant and widely distributed invasives» in temperate zones42. These species were Allolobophora chlorotica Savigny, 1893; Amynthas corticis Kinberg, 1867; Aporrectodea caliginosa Savigny, 1826; Ap. rosea Savigny, 1826; Ap. trapezoides, Dugès 1828; Eisenia andrei Bouché, 1972, Lumbricus terrestris Linnaeus 1758, L. friendi Cognetii, 1904, Octolasion cyaneum Savigny, 1826 and O. tyrteum Savigny, 1826 (Table 1; Figure 1). It is the first time Am. corticis is reported in Uruguay, although this species is highly associated to Am. gracilis43, which was already reported in Montevideo in the early studies conducted by Cordero12.
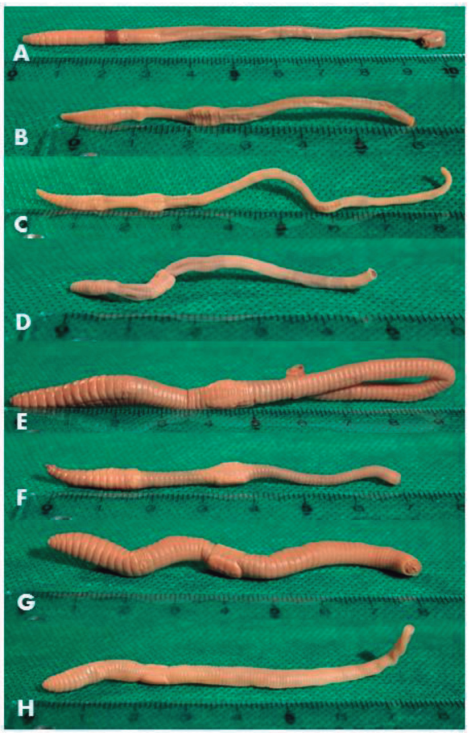
Figure 1: Ventral/latero-ventral view of exotic earthworm specimens fixed in formaldehyde and preserved in 80 % alcohol: A) Amynthas corticis, B) Allolobophora chlorotica, C) Aporrectodea caliginosa, D) Ap. rosea, E) Lumbricus terrestris, F) L. friendi, G) Octolasion tyrtaeum, H) O. cyaneum. The rear end has been cut for DNA extraction. Numbers on the ruler correspond to centimeters. Photos: Gerardo Bentancur.
In the case of the genera Allolobophora, Amynthas, Aporrectodea and Octolasion, BLAST similarity analysis showed that COI fragments had over 97 % similarity with those sequences annotated in GenBank. Conversely, the genus Lumbricus showed lower similarity with the annotated sequences, between 74 % and 87 % (Table 1). Only three specimens of the genus Lumbricus were DNA sequenced in this study, and the obtained sequences were rather short, probably due to degraded DNA (data not shown). Hence, DNA sequence data are not conclusive and further investigation is needed to confirm if these low similarity levels correspond to any differentiation involving the presence of cryptic species, as described for this genus in other invaded countries44)(45. Future studies should provide additional specimens, including COI sequences, as well as a complementary nuclear marker, such as Histone 3 or ITS246, so as to get a robust phylogeny analysis and multi-locus species delimitation.
Based on multigene analysis, Martinsson and Erseus44 found genus Lumbricus to be monophyletic, with maximum support in their H3 tree and some support in the COI tree, using sequences of European Lumbricus castaneus, L. festivus, L. herculeus, L. rubellus, and L. terrestris. They suggested that L. rubellus morphospecies is composed of seven cryptic species. Their analysis also confirmed the previously suggested division between L. terrestris and L. herculeus, as two different species47.
According to size range attributed to L. herculeus47, there was some suspicion that small Lumbricus specimens in this study could belong to this species (Figure 1 E and F). However, this fact was not confirmed by the BLAST similarity analysis. Instead, some similarity appeared with L. friendi, but as this species sequence is unpublished, no certain conclusions can be drawn from that similarity, although external morphological characters coincide with L. friendi description (Figure 1 F; 17).
Two cryptic lineages of Ap. caliginosa were found (L2 and L3, Table 1), which have also been reported for Europe and North America48. Porco and others48 highlight the importance of these cryptic lineages to detect earthworm invasive patterns, which morphological features could mask. For instance, in the present study Ap. caliginosa L2 was only found in Montevideo, while Ap. caliginosa L3 was found in both provinces, being the first report of this species for Paysandú, which had previously only been found in the Uruguayan provinces of Montevideo, San José, Colonia and Treinta y Tres12. However, due to the low number and geographical concentration of samples, particularly in Paysandú, it is possible to find Ap. caliginosa L2 in further samplings in this province. Still, the absence of Ap. caliginosa L2 from this province could be a hypothesis for future studies, being the present study a possible preliminary survey.
Changes in the earthworm community composition as a consequence of a certain use and management of the soil are expected, since different ecological groups are affected differently by agricultural activities. Species that feed on the surface and bury fresh organic matter into greater depths in a vertical galleries system (anecic species), such as L. terrestris and L. friendi, are more affected by agriculture management than other species that live and feed within the soil (endogenous species) such as All. chlorotica, Ap. caliginosa, Ap. rosea, Ap. trapezoides, O. cyaneum and O. tyrtaeum49. Earthworms that only live superficially (epigeic species), are the most affected by tillage, but can survive under mulch by feeding on plant debris49. Epigeics found in this study were E. fetida and Am. corticis, although not typical of agriculture land, they were most probably added to the soil with organic matter incorporation, since both can be found in compost piles. Since they show different sensitivity to management according to their ecological group, it is interesting to use earthworms as bioindicators of soil quality not only in terms of density and biomass16)(50)(51)(52 variations but also to reach to species level and take advantage of the information provided by the possible changes in the community and ecological groups15.
In summary, molecular techniques along with morphology allowed a fully identification of several species found in two different localities. The possibility of identifying species by DNA barcoding is more accessible22)(53 with a repository of public sequences (e.g. GenBank). However, it relies on the fact that the sequences have previously been uploaded and published. The limited number of Uruguayan native species sequences available in GenBank is a major constraint, with only two species having its COI sequence annotated in RefSeq. Overcoming this limitation will require a detailed morphological study with a precise identification of morphospecies, generating the corresponding sequences and thus, expanding the native species database.
Adding more native species to the online database represents a future challenge. Hence, it is essential to encourage earthworm taxonomy training, since the adoption of molecular techniques does not dismiss the traditional taxonomy based on morphological characteristics. This preliminary study could kick-start a local innovative research program, since there are limited records of earthworm samplings in Uruguay and no identification of species by DNA sequences from national studies. Now that earthworms are recognised as a heterogeneous group, with different responses to agricultural management and potential to provide different and complementary ecosystem services, to be able to track changes in earthworm community composition, will help in the search of more sustainable management production systems, both by preserving biodiversity and by taking advantage of the ecosystem services earthworms may provide.