Introduction
Eragrostis plana Nees, of the Poaceae family, is a densely tufted, allochthonous and perennial C4 grass. It grows to a height of 0.5 to 1 m, and forms dense clumps due to the intense tillering. The leaves of this species are characterized by its high fiber content.
Known as lovegrass, this species is native of south-west Africa and, nowadays, it is an invasive species in many parts of Asia, India, South America and the United States1. It has been introduced in Brazil, where it is a troublesome weed of natural pasture, rangeland, and roadsides2. It was accidentally introduced in the state of Rio Grande do Sul in the 1950s as a contaminant in Chloris gayana Kunth seed shipments2, where it became known as ‘capim annoni’ as a reference to those responsible for its introduction in the State. Since then, E. plana has become the most abundant and aggressive invasive species in the Pampa biome, responsible for widespread economic impacts on traditional livestock production by modifying the structure of plant communities and altering the ecological balance. These degradation processes not only lead to biodiversity loss but also reduce important ecosystem services provided by grasslands, such as carbon storage and water infiltration in soil; erosion control; and forage production3. According to Medeiros & Focht4, approximately 20 % of grassland in Rio Grande do Sul is already infested with lovegrass, which corresponds to an area of 3.1 million hectares. Its expansion into different regions occurs from the animals drive and car traffic5.
This species is highly prolific and its hardiness and adaptability to poor soils have allowed great multiplication and invasive behavior6. Another important aspect regarding lovegrass is its high potential for seed production of small size and high germination capacity4, with a single individual able to produce around 300,000 seeds7. These seeds have high viability and can last for long periods as part of the seed bank in the soil. This mechanism enables the regeneration and reestablishment of new populations in response to any soil disturbance4. Recovery and germination of seeds buried at different depths in the soil profile (surface, 2.5, 5, 10 and 20 cm) were described by negative exponential models4. Germination of surface seeds, after two years, was 4.5 %, and 40.3% for seeds 20 cm deep. According to models, 0.1% of the seeds on the surface survive after five years and, for those buried at 20 cm, 0.01 % is still viable after 24 years. This shows that the deeper the seed, the higher the preservation of germination capacity. Due to these characteristics, this species has become invasive and difficult to eradicate2)(8.
Among other factors, aggressiveness in dispersion and dominance of lovegrass is attributed to its high adaptability to different environmental conditions, capable of developing well and fast even in unfavorable conditions. However, the mechanisms of adaptation and tolerance to external factors are not understood. Knowing the physiological aspects of its tolerance to adverse conditions can be beneficial for management strategies of invasive species9. Information about the ecological aspects that affect lovegrass germination is scarce10, and no studies were found on seeds collected in Rio Grande do Sul that consider these aspects. Therefore, the objective of this study was to investigate how temperature, luminosity, and osmotic potential can influence the germination of E. plana seeds.
Material and Methods
The study was conducted in the Laboratory of the Fundação Estadual de Pesquisa Agropecuária, Centro de Pesquisa em Florestas, Santa Maria, RS, as part of a project that searches for alternatives to control the dispersion of Eragrostis plana in the Pampa biome.
E. plana seeds were collected in spring 2014, in the municipality of Hulha Negra, RS, in the Campanha physiographic region, which is a highly infested area. Seeds were conditioned in glass bottles in a refrigerator (ca. 4 ºC) for five months until the experiments were conducted.
A previous test found that there was no need for any kind of dormancy break for seeds (data not shown), therefore, only a slight disinfection with commercial hypochlorite (2.5 %) and water at a 1:1 (v/v) ratio for 5 minutes and washing with distilled water were performed before each test.
Germination tests for all experiments were carried out in transparent plastic boxes (11 x 11 x 3.5 cm), with four replicates of 25 seeds in each treatment.
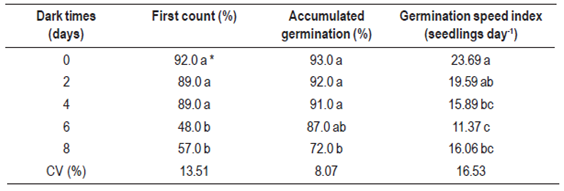
To evaluate the effect of a period of dark incubation before light exposition, we used five treatments consisting of time (days) in the dark (0; 2; 4; 6; 8 days), i.e. every two days a new batch (treatment) of seeds was exposed to a photoperiod room. Darkness was achieved using brown packaging that do not allow the passage of light. Five milliliters of distilled water were added to each replicate as well as two milliliters every four days to maintain moisture. The experiment was carried out in a climatized room with a photoperiod of 12 h of light and photon fluence rate of 35 µmol m-2s-1, provided by cool white fluorescent lamps. To obtain the data for germination speed index calculation, packages were opened briefly to assess at the same time each day.
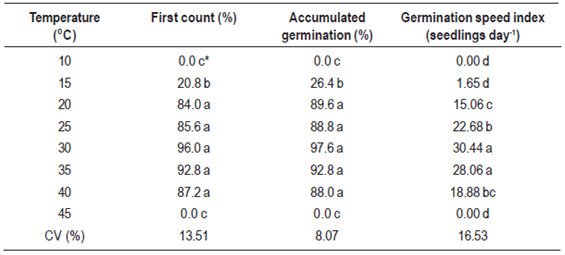
The effect of temperature on seed germination was evaluated by using eight different temperatures (10; 15; 20; 25; 30; 35; 40; 45 ºC). Five milliliters of distilled water were added to each replicate as well as two milliliters every four days to maintain moisture. The experiment was carried out in a BOD with artificial photoperiod (12 hours light) and light intensity of photon fluence provided by four fluorescent lamps 20W, with radiant flux density at the time of crating of 15 µmol m-2 s-1.
To evaluate the effect of different levels of osmotic potential in the substrate on seed germination, aqueous solutions of polyethylene glycol (PEG 6000) and sodium chloride (NaCl) were used at the following osmotic potential: 0; -0.2; -0,4 and -0.8 MPa. To obtain the PEG and NaCl solutions, the table proposed by Richards11 was used, where salt amounts were diluted separately in 200 mL of distilled water: 0.946, 1.892 and 3.784 g NaCl and 23.914, 35.668 and 52.382 g of PEG with osmotic potential of -0.2, -0.4 and -0.8 MPa, respectively. Five milliliters of PEG solution were added to each replicate as well as two milliliters every four days to maintain moisture. The experiment was carried out in a climatized room with a photoperiod of 12 h of light and photon fluence rate of 35 ìmol m-2s-1, provided by cool white fluorescent lamps.
The germinative capacity was evaluated through the following characteristics:
- Germination speed index (GSI): the test was implemented in the same way as the germination pattern test. Germinated seeds were counted daily, at the same time, during the 14 day period, remoistening the substrate every four days. The criterion for germination was root protrusion (2.0 mm). The speed index germination was calculated as the mean of the values obtained for the four replicates of 25 seeds using the formula:
GSI= G1/N1 + G2/N2 + ... + Gn/Nn12,
where: GSI = germination speed index G1, G2, Gn = number of germinated seeds computed in the first count, the second count until the last count; N1, N2, Nn = number of days from sowing to first, second, and last count.
- First count: was conducted jointly with the standard test of germination, determining the percentage of germinated plantlets seven days after the start of the test, as described by Rules for Seed Analysis (RAS)13.
- Accumulated germination: the count of the accumulated germination was carried out 14 days after seeding, as recommended by the RAS13. The results were expressed in the average percentage of germinated plantlets.
The experimental data were subject to analysis of variance. The mean differences were compared by Tukey’s multiple range test (P < 0.05) with the help of the statistical program SISVAR 5.614.
Results and Discussion
Our results demonstrate that all the evaluated abiotic factors had an influence on E. plana germination (Tables 1, 2, 3, 4), indicating ranges of light, temperature, and osmotic potential that promote a reduction in seed germination.
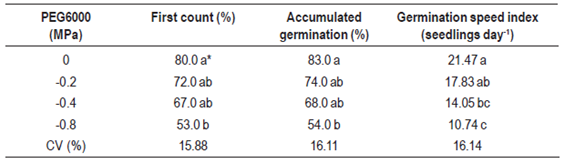
Table 3: Germination of Eragrostis plana Nees seeds under different osmotic potentials in polyethylene glycol (PEG 6000) solution.
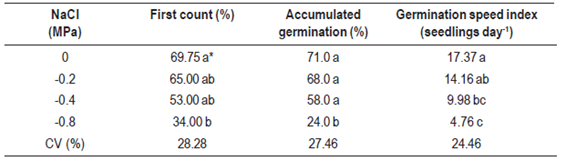
Table 4: Germination of Eragrostis plana Nees seeds submitted to different osmotic potentials in NaCl solution.
Lack of light inhibited lovegrass germination, although seeds could be stimulated for germination by very brief expositions to light15. Our results showed that, even with this small interference at evaluations, dark permanence inhibited germination, especially for seeds that remained for more than six days in the dark and for germination speed index after four days in the dark (Table 1), i.e. darkness had a greater effect on the germination speed index than on the effective germination rate. In addition, seedlings that germinated at six and eight days in the dark showed visible symptoms of etiolation. Light quality provides a detection mechanism for regeneration of seed germination in many ecosystems16. This confirms the opportunistic behavior of lovegrass, since it grows, for example, in naked soil or roadsides, where the frequency and scale of disturbance leave the system in constant anthropogenic disclimax17.
Some studies have indicated that shading decreases plant height of E. plana and decreases tillering, thus reducing the clump diameter and possibly decreasing seed production18)(19. However, these previous experiments do not provide information on the effect of shading on seed germination. Bittencourt and others10 evaluated the effect of absence of light on the germination of lovegrass seeds; however, these authors did not present specific responses of the different dark periods after the germination stimulation. On the other hand, our results indicate that the lack of light delayed lovegrass germination, which can be verified by the difference between the first count and accumulated germination, associated to the decrease in germination speed rate. This information may be useful in programs which contain this invasive species; as an example, through the consortium of agroforestry systems, which would promote the formation of shaded areas in the fields that are less susceptible to lovegrass infestation.
In agreement with these results, a study involving silvicultural shading using acacia forests (Acacia mearnsii) showed that, after four years of implantation, shading not only reduced infestation of lovegrass, but also suppressed the rest of the vegetation. In the second phase of the aforementioned study, thinning was performed on acacia plants by cutting some planting lines to allow light to enter; after thinning, few annoni grass plants were vigorous, although the authors recorded the presence of other invasive plants20.
In regard to temperature, E. plana seeds were capable of germinating at different times of the year due to their ability to tolerate a wide temperature range (Table 2). The optimal temperature for faster and more efficient germination was approximately 30 °C, although temperatures between 20 and 40 °C were favorable. However, the extremes (below 10 and above 40 ºC) were highly inhibitory for lovegrass germination (Table 2). Our results corroborate those presented by Bittencourt and others10. The optimal temperature is one that enables the most efficient combination between percentage and germination rate.
Seeds that tolerate a wide temperature range favor their propagation and the subsequent colonization of new areas15)(21. In our study, lovegrass presented high accumulated germination rates, above 88%, between 20 °C and 40 ºC (Table 2), which explain the occurrence of this species in different seasons. To most species, the optimal germination temperature, at which greatest germination is achieved in the shortest time, is found between 15 and 30 °C; maximum temperatures range between 35 and 40 °C and the minimum temperature is the freezing point15. Above the maximum temperature, seeds generally die in a few days and below the minimum temperature, seeds do not germinate for a reasonable time period.
Temperature can regulate germination in three ways: determining the capacity and rate of germination; removing primary or secondary dormancy; and inducing secondary dormancy15. It is known that the effect of temperature on germination is particularly important for populations ecology and that the higher the temperature range, the wider the geographic distribution of the species22. The high dispersibility of E. plana can also be attributed to its tolerance to a wide temperature range, which favors seed propagation in different seasons of the year.
Due to the importance of water availability for seeds germination, some reagents were used to simulate the effects of water restriction, especially polyethylene glycol (PEG 6000) and sodium chloride (NaCl). Results showed that decreasing osmotic potential triggered by PEG 6000 decreased the lovegrass germination percentage (first count and accumulated germination) and also the germination speed index (Table 3). In the same way, when NaCl was applied as an agent of osmotic potential, seed germination was significantly affected (p > 0.05), especially when a lower osmotic potential was induced (-0.8 MPa).
Comparing both reagents, germination speed was reduced by 50 % for the lower osmotic potential triggered by PEG, while in the lower potential, induced by NaCl, germination speed was reduced fourfold (Tables 3 and 4), showing that osmotic stress caused by NaCl in E. plana was more aggressive than that caused by PEG. Although the refill of the solutions of the respective treatments could have slightly changed the water potential during the experiment, results showed significant differences between treatments. Andréo-Souza23 investigated jatropha seeds (Jatropha curcas L.) and found that germination speed was the first parameter affected by a decrease in water availability, resulting in a longer time to complete the germination process. On the other hand, Dantas24 used polyethylene glycol (PEG 6000) and NaCl in safflower seeds and noted that the percentage of germination was more affected in treatments where osmotic stress was induced by PEG 6000 than in those by NaCl.
Water is needed to hydrate seed tissues and to increase respiratory and metabolic activities, releasing energy and nutrients to sustain embryo growth15. A higher concentration of salts in the soil reduces its water potential, resulting in lower water absorption by plants. Decrement in germination can also be attributed to a lower mobilization of reserves, reduced synthesis, and enzymatic activity or changes in cell turgor15. Carneiro25 evaluated water and salt stress in sunflower seedlings and found that levels of osmotic potential of 0.4 MPa did not affect the physiological performance, but more severe stress at a potential of -0.8 MPa reduced growth and antioxidant capacity of the seedlings, which gives less tolerance to water and osmotic stress.
Conclusions
Periods of light absence, more than six days after induction of germination, promoted a 22.5 % reduction on the Eragrostis plana germination rate. Germination of lovegrass was completely inhibited at temperatures below 15 °C and above 40 °C. Osmotic potentials below -0.8 MPa restricted the germination of lovegrass, especially in the presence of NaCl.











 Curriculum ScienTI
Curriculum ScienTI