Serviços Personalizados
Journal
Artigo
Links relacionados
Compartilhar
Odontoestomatología
versão impressa ISSN 0797-0374versão On-line ISSN 1688-9339
Odontoestomatología vol.24 no.39 Montevideo jun. 2022 Epub 01-Jun-2022
https://doi.org/10.22592/ode2022n39e312
Update
Pathophysiology of COVID-19
1Cátedra de Fisiopatología, Facultad de Odontología, Universidad de la República, Uruguay. brunomanta@odon.edu.uy.
Coronavirus disease is a respiratory infection caused by the SARS-CoV-2 virus, which causes a cascade of systemic events, affecting various organs and tissues. Understanding the pathophysiology of COVID-19 is essential to treat patients and understand the causes of the complications in a significant number of recovered patients. This article presents a review of the effects of infection on various organs and systems that will be useful as reference material for healthcare professionals and medical students. To this end, a literature search was conducted in PubMED, Scielo, Google Scholar, Cochrane, and Springer Link portals, as well as in the pre-publication scientific repositories bioRxiv (“bioarchives”) and medRxiv (“med-archives”) databases. From about 200,000 papers, 100 articles were selected for this review based on their relevance or suggestions from experts in the field.
Keywords: SARS-CoV-2; patophysiology; COVID-19; ACE2
La enfermedad por coronavirus es una infección respiratoria causada por el virus SARS-CoV 2, el cual genera una cascada de eventos sistémicos, afectando diferentes órganos y tejidos. El entendimiento de la fisiopatología del COVID-19 es indispensable no solo al momento de brindar tratamiento a los pacientes, sino que también para comprender las causas de las complicaciones que presentan un número importante de pacientes recuperados. El objetivo de este trabajo es presentar una revisión actualizada de los efectos de la infección en diferentes órganos y sistemas principales que sea de utilidad como material de referencia para profesionales y estudiantes de la salud. Para ello se realizó una búsqueda bibliográfica en los portales PubMED, Scielo, Google Scholar, Cochrane y Springer Link, así como en las bases de repositorios científicos pre-publicación bioRxiv (“bioarchives”) y medRxiv (“med-archives”) y sobre un total de cerca de 200 mil artículos, se seleccionaron 100 artículos para esta revisión en base a su relevancia o sugerencias de parte de profesionales especializados.
Palabras claves: SARS-CoV-2; fisiopatología; COVID-19; ECA2
A doença coronavírus é uma infecção respiratória causada pelo vírus SARS-CoV-2, que gera uma cascata de eventos sistêmicos, afetando diferentes órgãos e tecidos. Compreender a fisiopatologia da COVID-19 é essencial não apenas no tratamento de pacientes, mas também para compreender as causas das complicações que um número significativo de pacientes recuperados apresenta. O objetivo deste trabalho é apresentar uma revisão atualizada dos efeitos da infecção em diferentes órgãos e principais sistemas que seja útil como material de referência para profissionais de saúde e estudantes. Para isso, foi realizada uma pesquisa bibliográfica nos portais PubMED, Scielo, Google Scholar, Cochrane e Springer Link, bem como nos repositórios científicos de pré-publicação bioRxiv (“bioarquivos”) e medRxiv (“arquivos med”). Num total de cerca de 200 mil artigos, 100 artigos foram selecionados para esta revisão por sua relevância ou sugestões de profissionais especializados.
Palavras claves: SARS-CoV-2; fisiopatología; COVID-19; ECA2
Introduction
COVID-19 disease is caused by SARS-CoV-2 infection, a new type of coronavirus that appeared in late 2019 in China,1 unleashing the first known coronavirus pandemic in modern times. Although SARS-CoV-2 infection was initially described as an “atypical pneumonia,” extrapulmonary manifestations were quickly identified, particularly in patients with comorbidities.2-6 Most of the pathophysiological manifestations of this virus occur because its entry point is the angiotensin-converting enzyme 2 (ACE2), a key element of the renin-angiotensin system (RAS).7,8 In addition, the pathophysiological bases that determine that preexisting conditions like cardiovascular disease (CVD), diabetes, or obesity predispose patients to a worse prognosis are also linked to alterations in ACE2 levels or RAS function.9-12
Methodology
A literature search was conducted on scientific articles in English and Spanish published between January 2020 and May 2021 using the keywords “COVID,” “COVID-19,” “SARS-CoV-2,” and “coronavirus” in PubMED, Scielo, Google Scholar, Cochrane, and Springer Link, as well as in the pre-publication scientific repository databases bioRxiv (“bioarchives”) and medRxiv (“med-archives”). Between January 2020 and May 31, 2021, over 150,000 articles were published. Therefore, for this article, we considered mainly reviews in medical, dental, or generalist journals of broad dissemination, information centralized by international agencies such as WHO, CDC, reports published by the Honorary Scientific Advisory Group (GACH13), the Uruguayan Interdisciplinary Group for COVID-19 Data Analysis (GUIAD14), as well as specific articles suggested by national and international experts.
Biology of SARS-CoV-2
Coronaviruses are classified into four groups named α, β, γ, δ.15,16 At least seven species infect humans: two from the α-family, known as HCoV-229E and HCoV-NL63, and five from the β-family: HCoV-HKU1, HCoV-OC43, SARS (Severe Acute Respiratory Syndrome Coronavirus, now called SARS-CoV-1), MERS (Middle East Respiratory Syndrome, now called MERS-CoV) and the recently discovered SARS-CoV-2.16 HCoVs infect the upper respiratory tract of children and adults and account for a proportion of seasonal mild respiratory infections diagnosed each year.15
SARS-CoV-2 is a single-stranded RNA positive polarity virus, with a genome of 30,000 base pairs, as expected for coronaviruses. It harbors few genes, including nonstructural and structural proteins. Structural proteins are those that form the viral capsid and include the N protein (nucleocapsid) that binds to the genetic material of the virus, the E and M proteins that are anchored to the membrane, and the S protein (spike) that is the key to virus infectivity since it carries the “key” to open the “lock” of the cell membrane.17
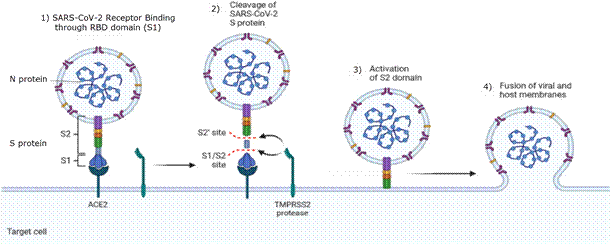
SARS-CoV-2 uses the same mechanism of infection as other coronaviruses, based on the S protein recognizing ACE2 (Figure 1, step 1). The S protein is a glycoprotein consisting of two domains, the S1—with the region known as RBD, which binds to ACE2—and the S2, which has the membrane fusion machinery that allows the virus to enter the cell. ACE2 binding causes structural changes in S1, leaving cleavage sites exposed to proteases present in the cell membrane like transmembrane serine protease 2 (TMPRSS2) or furin.17 This protease activity cleaves between S1 and S2 (Figure 1, step 2), which unlock the membrane fusion machinery present in S1 domain (Figure 1, step 3) allowing the virus to fuse its membrane to the cell membrane, and virus entry through endocytosis (Figure 1, step 4).
Once inside the cell, the virus cycle is similar to other RNA viruses.17-19
Pathophysiology of COVID-19
Several preexisting diseases or conditions are considered COVID-19 comorbidities mainly because they share pathophysiological features with COVID-19 disease.2,9,20-24 Below we present a brief summary of the pathophysiological basis of COVID-19 disease and the most common comorbidities, including key references that guide readers to additional references on each topic.
The renin-angiotensin system
The best-known function of the renin-angiotensin system (RAS) is to maintain homeostasis between vessels, blood, and body fluid volume.25,26 This system is intrinsically associated with cardiac output, blood pressure, and regulation of electrolyte balance.27) The main elements of RAS are angiotensinogen, angiotensin 1, angiotensin 2, and theangiotensin-converting enzymes (ACE) 1 and 2. In our circulation, angiotensinogen—a glycoprotein secreted mainly by the liver—is proteolyzed by the action of renin, secreted by the kidney in response to decreased blood pressure.25 The product is a 10 amino-acid peptide called angiotensin 1, which is converted to angiotensin 2 (8 amino acids) by ACE1. ACE1 is a membrane protein expressed mainly in lungs, intestinal epithelium, kidney, and bladder. Angiotensin 2 performs endocrine functions in several organs by binding to specific membrane receptors (AGTR1 and AGTR2), causing:
1) contraction of vascular smooth muscle,
2) stimulation of vasopressin secretion by the pituitary gland (antidiuretic hormone),
3) stimulation of aldosterone secretion by the adrenal cortex of the kidney, and
4) increase reabsorption of water and sodium reabsorption by nephrones.
Respiratory system involvement
Since SARS-CoV-2 is transmitted through aerosols or microscopic droplets, (29 it is expected to have tropism for tissues in the nasopharyngeal cavity and respiratory tract. This tropism is given by ACE2 expression in these tissues.12 SARS-CoV-2 infection of the respiratory system occurs in three phases. The first phase occurs in the nasopharyngeal cavity, infecting some cell types (see section 4.7) but does not induce a vigorous immune response, and is generally the type of infection present in asymptomatic individuals. The second phase involves infection of the major airways, bronchi, and bronchioles; it manifests with pulmonary inflammation symptoms and may occur with or without hypoxia. The third phase involves infection of the gas exchange structures, the alveoli, mainly formed by two cell types of epithelial origin called type I and II pneumocytes.30 Type I pneumocytes have a classic epithelial morphology, while type II are cuboidal and smaller, and contain organelles called “lamellar bodies” that secrete pulmonary surfactant. Without this surfactant, the alveoli would collapse after exhalation27 (Figure 2). Alveolar homeostasis is maintained by a network of resident cells, including epithelial cells, endothelial cells, and leukocytes.31 Resident alveolar macrophages and epithelial cells form a critical barrier in the lung (Figure 2, left). The infection of a type II pneumocyte increase the expression of genes associated with antiviral response, such as interferons and certain interleukins, and decrease the expression of genes responsible for surfactant production.32 These signals activate immune cells in the alveoli, such as macrophages, and recruit others from circulation, such as neutrophils31 (Figure 2, right). Infected cells develop a high viral load30 and trigger a cell death program called “pyroptosis”, which involves the massive release of inflammatory mediators.33 This triggers a cascade of events that increases type I pneumocyte damage, with the subsequent breakdown of the alveolar barrier and infiltration of plasma protein and cellular components. The alveolus damaged by the immune response begins to fill with a mixture of plasma exudate, dead cells, viral particles, inflammatory cells, and fibrin, increasing the volume of the interstitium between capillaries and alveolar chamber (Figure 2, right). As a consequence, gas exchange is compromised, which ultimately leads to the associated respiratory dysfunction that gives its name to the disease, SARS: severe acute respiratory syndrome.30,31,34 Additionally, the immune response associated with the infection can trigger a response known as a “cytokine storm,” which is a cascade of inflammatory events that generate a sustained hyperinflammation that can cause hypercoagulability in the microvasculature and lead to tissue injury, disseminated intravascular coagulation, and multiorgan failure.35,36
At first, it was thought that children and young people are less susceptible than older adults to infection because the expression of the ACE2 receptor in lung tissue increases with age, increasing the density of targets for the virus. However, there are no significant differences in ACE2 levels between ages and sexes.12 The most likely explanation for this apparent contradiction suggests that reduced ACE2 expression in the membrane of type II pneumocytes increases angiotensin 2 levels to the detriment of angiotensin 1-7 formation with aging. This exaggeratedly triggers proinflammatory pathways and predisposes older patients to severity of acute lung injury and COVID-19 mortality.37
Secondary bacterial infections are common in patients with COVID-19, particularly in those requiring mechanical ventilators or intubation.1 This might be the case because infection, and the associated damage, modify the community of microorganisms (microbiome) that reside in the airways, enabling the proliferation of opportunistic pathogens.38 In any case, bacterial pneumonia is a significant cause of complications in patients with COVID-19, leading to increased mortality rates.39
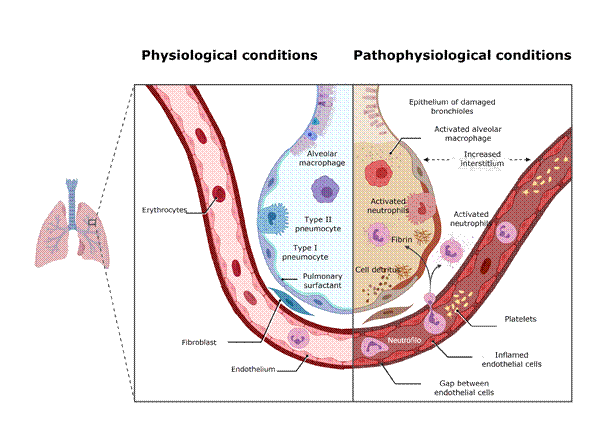
Figure 2: Representation of a pulmonary alveolus in physiological conditions and after SARS-CoV-2 infection.
The left half of the figure represents the structure and cells of an alveolus in physiological conditions; the right half represents the changes caused by viral infection. See the text for a full description. Figure created with Biorender.com
Effects of infection on the cardiovascular system
SARS-CoV-2 infection lethality is strongly associated with the patient’s age and the type of treatment, ranging from less than 1% for children to over 10% in patients over 70 years old.39,40 However, in any age range, preexisting cardiovascular disease increases the risk of death 3-4 times.10,41 In fact, the main complications and causes of death in patients with COVID-19 are thrombotic events, like venous thromboembolism, or disseminated intravascular coagulation.19,42 The pathophysiological basis for this is the existence of feedback loops between the preexisting disease and the pathology caused by the infection. On the one hand, COVID19 causes significant hematological alterations,35,36,43 some typically associated with the antiviral response, such as leukopenia (present in 80% of hospitalized patients), thrombocytopenia (30%). These are most probably related to the associated immune response, such as the increase of inflammatory cytokines IL-6, IL-2, IL-7, interferon-γ, TNF-α, and elevated plasmatic levels of thrombotic risk markers C-reactive protein, D-dimer and procalcitonin.36,44,45 In addition, a massive infection significantly reduces ACE2 expression or presence in the cell membrane.7 This deregulates the RAS system because angiotensin 2 is not degraded, causing a chronic hypertensive condition which, added to the prothrombotic state, significantly increases the risk of thromboembolism.19 One of the main reasons why patients with cardiovascular disease are at higher risk for COVID-19 and also have a worse outcome is that many diseases such as hypertension, diabetes, coronary heart disease, among others, result in chronic RAS deregulation.10,41
Beyond initial doubts about the potential effect of the virus on the myocardium,19 recent results based on tissue sequencing in autopsies show that direct infection is not a likely cause of the heart failure commonly described in terminally ill patients.32 In general, these effects on the cardiovascular system and the heart are considered secondary to the systemic inflammation produced by the virus, i.e., they are the outcome of endothelitis and microthrombus formation. The endothelium is a central player in cardiovascular physiology, and there is clear evidence that it has a significant role in the pathogenesis of COVID-19.46
Effects of infection on the endocrine system.
Type 2 diabetes (T2DM) is closely associated with the typical Western lifestyle and diet, especially with the obesity pandemic that began several decades ago,6,47,48 which is also prevalent in Uruguay.49 T2DM is highly prevalent in some regions of the world and in Uruguay it affects >20% of the population 50. Diabetes was identified early on as having a significant impact on the COVID-19 pandemic, increasing the chances of COVID-19-associated hospitalizations and deaths.6,10,23 People with T2DM are at increased risk of infection due to immune response deficiencies associated with chronic metabolic disease.23,51 It has also been reported that patients with diabetes have a higher ACE2 expression in bronchi and alveoli, suggesting that they are more prone to SARS-CoV-2.52
Potential pathogenic links between COVID-19 and diabetes mellitus include effects on glucose homeostasis, inflammation, altered immune status, and RAS activation.9 Additionally, the infection can cause ketoacidosis and an increase in blood glucose levels due to immune dysregulation or the steroids administered to hospitalized patients23 (e.g., glucocorticoids). In this context, managing patients with diabetes during the infection requires special attention since routine medication to control their condition may be incompatible with the necessary COVID-19 treatment.9,51
The COVID-19 epidemic has led to an increase in new-onset type 1 diabetes (T1DM) cases.53,54 It was originally considered that direct β-cell infection was responsible for the T1DM increase. However, autopsies seem to indicate that the pancreas is not a relevant reservoir for the virus and that it is unlikely that SARS-CoV-2 infect pancreatic cells directly.32 Consequently, several mechanisms have been proposed to explain autoimmune insulitis and β-cell destruction. This is an issue of utmost healthcare importance as medical evidence indicates that patients who have recovered from COVID-19 are 40% more likely to develop T1DM.55
Regarding the function of other organs in the endocrine system, some reports indicate that SARS-CoV-2 infection causes both hypothyroidism and thyrotoxicosis and deficiencies in the function of parathyroid and adrenal glands.56,57 However, autopsies suggest failure due to thrombotic events42 and not by direct infection of glandular tissues by the virus.32
Effects of infection on the digestive system
Approximately 20% of patients with the disease present gastrointestinal symptoms such as nausea, vomiting, and diarrhea,58 percentage which rises to 50% in hospitalized patients.59 The virus’s genetic material can be detected in the feces of infected patients, even if they are asymptomatic.60 There is conclusive evidence that enterocytes are directly infected by SARS-CoV-2,61 which is consistent with the fact that ACE2 expression in the intestinal epithelium is the highest in the body.12 Direct infection of enterocytes explains why the most critically ill patients present symptoms similar to inflammatory bowel diseases such as irritable bowel syndrome and Crohn’s disease.58 This includes the increased presence of biomarkers for disrupted gut permeability62. These include increased IL-17-producing TH17 cells and high cytotoxicity of CD8+ T cells in the peripheral blood, which are thought to play an essential role in a cytokine storm. Therefore, the proinflammatory features seen in the lungs also appear to be present in critically ill patients’ gut.60 In addition, changes in the intestinal microbiota have been reported due to the infection.63 SARS-CoV-2 infection also appears to affect the exocrine pancreas, manifesting as pancreatitis with altered blood amylase and lipase levels, although the etiological relationship between infection and pancreatitis is inconclusive.64
Central nervous system involvement
Approximately 30% of patients have neurological symptoms, ranging from headaches to severe complications such as cerebrovascular infarction. Pathophysiology manifestation of COVID-19 in the nervous system may be due to three factors:
1) chronic inflammation,
2) thrombotic events induced by the underlying coagulopathy,
3) direct infection of nervous system cells.
Effects of the infection on the oral cavity and dental implications
Since the beginning of the pandemic, it’s known that the saliva of people infected with COVID-19 may contain high levels of SARS-CoV-2. In fact, much of the prevention efforts have focused on halting the spread of the virus by aerosols and droplets exhaled by carriers.29 As a result, the dental practice has been severely affected.67,68 The virus that can be detected in the saliva of people with respiratory symptoms possibly comes from nasal drainage or sputum expelled from the lungs. However, the presence of SARS-CoV-2 in the saliva of asymptomatic patients suggests that the virus may reside and replicate in extrapulmonary tissues of the upper airways.69 Huang et al. demonstrated that salivary glands and oral cavity epithelia could be infected by SARS-CoV-2 and transmit the infection to other organs.22 Using sequencing techniques, the authors demonstrated the presence of membrane receptors necessary for virus entry in all cell types in the oral cavity and the presence of the virus in tissue biopsies. They concluded that the mouth is an infection site and a reservoir for the virus. Probable hypotheses supporting dysgeusia and ageusia in COVID-19 include the possibility of damage caused by SARS-CoV-2 to salivary gland epithelial cells targeted by the virus due to ACE2 expression.70
Xerostomia is the most common clinical manifestation of SARS CoV-2 infection and is associated with a deregulated electrolyte balance resulting from deregulation of the RAS system,71 but it is not the only manifestation. Various lesions have been reported in the oral cavity of infected patients throughout these months: ulcers, erosions, blisters, vesicles, pustules, macules, papules, plaques, pigmentation, scabs, necrosis, petechiae, erythema, and spontaneous bleeding. Xerostomia and poor oral hygiene seem to be the main factors predisposing patients to lesions, caries, and periodontitis, especially in patients with prosthesis. In addition, opportunistic infections, stress, trauma (secondary to intubation), vascular compromise, and the hyperinflammatory response secondary to COVID-19 creates favorable conditions for oral lesions to develop.72-76
SARS-CoV-2 infection also has direct and indirect consequences on the oral microbiota.77,78 Hyposalivation poses a potential risk of secondary infection because it decreases the amount and concentration of essential components in the response against pathogens, such as lysozyme, mucins, lactoferrin, peroxidase, α-
defensins, β-defensins, and cystatins, among others.
“Post-chronic” COVID
At the time of writing, over 200,000 people will have recovered from COVID-19 in Uruguay. Based on the worldwide trend, over 50% of them will continue to have symptoms or complications weeks or months after discharge. This picture has been called “long-term COVID” or “post-acute COVID.” This is a huge challenge for medical and dental care, since we do not know what the response of these patients will be to pathologies in the course of their lives, from dental treatments to organ transplants.55,79 If we go by the effects of SARS-CoV-1, the highly related virus that caused an epidemic outbreak in early 2000 (see below), the results are not encouraging: a 12-year follow-up study of 25 surviving patients showed a marked increase in cases of hyperlipidemia, cardiovascular disease, and metabolic disorders,80 with no clear pathophysiological basis. Interestingly, dysbiosis of the intestinal and oral microbiota persists even several months after the resolution of the disease, which could contribute to the persisting symptoms.77,78,81-83 This suggests that there is scope for non-invasive therapeutic interventions, including probiotic, dietary, antibiotic, or antiviral treatments.7,84
Epidemiology of the SARS-CoV-2 pandemic
In the first two years of the pandemic, over 200 million cases of COVID-19 were reported worldwide, and it is estimated that over 6 million people died due to the infection. This made COVID-19 the fourth leading cause of death worldwide (4.4%) in 2020.85 The numbers of infected and deceased people exceed by several orders of magnitude those that occurred following the epidemic outbreaks of SARS-CoV-1 (2002-2003), which caused the death of 813 of the 8809 people diagnosed 86 and MERS-CoV (2012-2013) which caused 858 deaths.87 The number of deaths from these outbreaks is surprisingly low, especially considering that SARS-CoV-2 has a relatively low case fatality rate: ~10% for SARS-CoV-1, >30% for MERS-CoV, and 1-2% for SARS-CoV-2.88 There are biological, epidemiological, and social reasons that explain—at least in part—why the COVID-19 epidemic took on such magnitude and why it continues to develop.
First, some aspects of the biology of the virus make it unique. Evidence from cell cultures, animal models and autopsies suggests that SARS-CoV-2 is more effective in the infection process89 mainly because of minor but crucial differences in the S protein compared to its counterpart in SARS-CoV 1 and MERS-CoV.17 There is a second epidemiological aspect: SARS-CoV-1 and MERS-CoV infections almost exclusively led to symptomatic lower respiratory tract infections, where patients rapidly developed a clinical condition within a short period of time (2-7 days),34 making it possible to rapidly identify and isolate those infected. Today's most widely accepted view is that the COVID-19 pandemic escalated because a relatively high percentage of infected people are asymptomatic but still can transmit the virus.90-92 This is strongly supported by the mobility of the various virus strains since its journey from China to the entire world. This shows the importance of asymptomatic carriers and the enormous dispersion of contagious diseases in our highly interconnected world. Even in a small country with scarce international connectivity, such as Uruguay, genomic studies show that SARS-CoV-2 entered the country in mid-February 2020. During 2020 and 2021, viral strains continued to enter the country from different world regions, even though the number of international passengers arriving to the country during those year were extremely reduced.93-96
Finally, although we know that all three outbreaks were zoonotic, the origin of SARS-CoV-1 and MERS-CoV was quickly identified, which made it possible to manage the animal reservoir more efficiently. Beyond reasonable doubt about its origin, scientific evidence indicates that SARS-CoV-2 spread from bats to humans through an unknown mammalian intermediary.16 Another unique feature of SARS-CoV-2 is that its first outbreak occurred in a densely populated and interconnected city (Wuhan, China). Despite efforts to control the epidemic, it rapidly spread worldwide.1
However, this devastating epidemic was foreseeable, or at least likely. In an article published in 2007, Cheng et al. review the scientific evidence on the emerging risk of new public health crises caused by coronaviruses and conclude that: “Coronaviruses are well known to undergo genetic recombination, which may lead to new genotypes and outbreaks. The presence of a large reservoir of SARS-CoV-like viruses in horseshoe bats (...) is a time bomb.”97 For decades, we have been aware that our food production model and lifestyles expose us to emerging zoonotic diseases, many of which have triggered pandemics or emergencies, such as the 2003 health emergency caused by the H5N1 avian influenza virus, the pandemic declared as a result of the H1N1 swine influenza virus in 2009, or the global health emergency triggered by the 2014 Ebola outbreak. The next pandemic is “just around the corner” and is very likely to be once again the product of a viral disease of zoonotic origin that is transmitted to humans as a result of handling food animals. At the time of writing, a new strain of avian influenza (H5N8) has already spread to almost 50 countries, even causing human infections.98
Conclusions
The SARS-CoV-2 pandemic confronted us with public health challenges never seen before. However, despite the overwhelming number of deaths, the response of the health and scientific systems of certain countries was crucial to reduce the potential damage. In the process, we (re)discovered the value of experimental science, biomedicine, interdisciplinarity, and evidence-based decision making.99 In addition, the pandemic allowed—or forced—many scientists and health professionals to focus on their work on a single pathology, leading to the discovery of aspects of human physiology that were not fully characterized and, more importantly, to highlighting unknown features of the pathophysiological basis of chronic or infectious diseases we have been dealing with for decades.2,55,79,100
REFERENCES
1. Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 2020 Feb 20;382(8):727-33. [ Links ]
2. Gupta A, Madhavan MV, Sehgal K, Nair N, Mahajan S, Sehrawat TS, et al. Extrapulmonary manifestations of COVID-19. Nat Med. 2020 Jul;26(7):1017-32. [ Links ]
3. Tabary M, Khanmohammadi S, Araghi F, Dadkhahfar S, Tavangar SM. Pathologic features of COVID-19: A concise review. Pathol Res Pract. 2020 Sep;216(9):153097. [ Links ]
4. Hu B, Guo H, Zhou P, Shi Z-L. Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol. 2021 Mar;19(3):141-54. [ Links ]
5. Centers for Disease Control and Prevention. El COVID-19 y su salud. Internet. 2020 cited 2021 Apr 30. Disponible en:: Disponible en:: https://espanol.cdc.gov/coronavirus/2019- ncov/need-extra-precautions/people-with-medical-conditions.html [ Links ]
6. Stefan N, Birkenfeld AL, Schulze MB. Global pandemics interconnected - obesity, impaired metabolic health and COVID-19. Nat Rev Endocrinol. 2021 Mar;17(3):135-49. [ Links ]
7. Sriram K, Insel PA. A hypothesis for pathobiology and treatment of COVID-19: The centrality of ACE1/ACE2 imbalance. Br J Pharmacol. 2020;177(21):4825-44. [ Links ]
8. Ryan PM, Caplice N. COVID-19 and relative angiotensin-converting enzyme 2 deficiency: role in disease severity and therapeutic response. Open Heart. 2020 Jun;7(1). [ Links ]
9. Lim S, Bae JH, Kwon H-S, Nauck MA. COVID-19 and diabetes mellitus: from pathophysiology to clinical management. Nat Rev Endocrinol. 2021 Jan;17(1):11-30. [ Links ]
10. Bae S, Kim SR, Kim M-N, Shim WJ, Park S-M. Impact of cardiovascular disease and risk factors on fatal outcomes in patients with COVID-19 according to age: a systematic review and meta-analysis. Heart. 2021 Mar 1;107(5):373-80. [ Links ]
11. Logette E, Lorin C, Favreau CPH, Oshurko E, Coggan JS, Casalegno F, et al. Elevated blood glucose levels as a primary risk factor for the severity of COVID-19. medRxiv 2021.04.29.21256294. (preprint), 2021. Disponible en: https://www.medrxiv.org/content/10.1101/2021.04.29.21256294v1 [ Links ]
12. Li M-Y, Li L, Zhang Y, Wang X-S. Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infect Dis Poverty. 2020 Apr 28;9(1):45. [ Links ]
13. Uruguay. Presidencia de la República. GACH, 2020, disponible en https://www.presidencia.gub.uy/gach/ [ Links ]
14. GUIAD-COVID-19; 2020, disponible en https://GUIAD-COVID.github.io/ [ Links ]
15. Burrell CJ, Howard CR, Murphy FA. Coronaviruses. Fenner Whites Med Virol. 2017;437-46. [ Links ]
16. Morens DM, Breman JG, Calisher CH, Doherty PC, Hahn BH, Keusch GT, et al. The Origin of COVID-19 and Why It Matters. Am J Trop Med Hyg. 2020 Jul 22;103(3):955- 9. [ Links ]
17. Hartenian E, Nandakumar D, Lari A, Ly M, Tucker JM, Glaunsinger BA. The molecular virology of coronaviruses. J Biol Chem. 2020 Sep 11;295(37):12910-34. [ Links ]
18. Harrison AG, Lin T, Wang P. Mechanisms of SARS-CoV-2 Transmission and Pathogenesis. Trends Immunol. 2020 Dec 1;41(12):1100-15. [ Links ]
19. Nishiga M, Wang DW, Han Y, Lewis DB, Wu JC. COVID-19 and cardiovascular disease: from basic mechanisms to clinical perspectives. Nat Rev Cardiol. 2020 Sep;17(9):543-58. [ Links ]
20. Smith JC, Sausville EL, Girish V, Yuan ML, Vasudevan A, John KM, et al. Cigarette Smoke Exposure and Inflammatory Signaling Increase the Expression of the SARS CoV-2 Receptor ACE2 in the Respiratory Tract. Dev Cell. 2020 Jun 8;53(5):514-529.e3. [ Links ]
21. Xu J, Chu M, Zhong F, Tan X, Tang G, Mai J, et al. Digestive symptoms of COVID-19 and expression of ACE2 in digestive tract organs. Cell Death Discov. 2020 Aug 11;6(1):1-8. [ Links ]
22. Huang N, Pérez P, Kato T, Mikami Y, Okuda K, Gilmore RC, et al. SARS-CoV-2 infection of the oral cavity and saliva. Nat Med. 2021 Mar 25. [ Links ]
23. Wang J, Meng W. COVID-19 and diabetes: the contributions of hyperglycemia. J Mol Cell Biol. 2020 Dec 1;12(12):958-62. [ Links ]
24. Braun F, Lütgehetmann M, Pfefferle S, Wong MN, Carsten A, Lindenmeyer MT, et al. SARS-CoV-2 renal tropism associates with acute kidney injury. Lancet Lond Engl. 2020 Aug 29;396(10251):597-8. [ Links ]
25. Cano F, Gajardo M, Freundlich M, Cano F, Gajardo M, Freundlich M. Eje Renina Angiotensina, Enzima Convertidora de Angiotensina 2 y Coronavirus. Rev Chil Pediatría. 2020 Jun;91(3):330-8. [ Links ]
26. Pérez PC, Fernández LM, García-Cosio MD, Delgado JF. Sistema renina-angiotensina aldosterona y COVID19. Implicaciones clínicas. Rev Esp Cardiol Supl. 2020;20:27-32. [ Links ]
27. Robbins & Cotran Pathologic Basis of Disease - 10th ed. Internet. cited 2021 Jul 24. Disponible en:: https://www.elsevier.com/books/robbins-and-cotran-pathologic basis-of-disease/kumar/978-0-323-53113-9 [ Links ]
28. Wang M, Xiong H, Chen H, Li Q, Ruan XZ. Renal Injury by SARS-CoV-2 Infection: A Systematic Review. Kidney Dis. 2021;7(2):100-10. [ Links ]
29. Greenhalgh T, Jimenez JL, Prather KA, Tufekci Z, Fisman D, Schooley R. Ten scientific reasons in support of airborne transmission of SARS-CoV-2. The Lancet. 2021 May 1;397(10285):1603-5. [ Links ]
30. Mason RJ. Thoughts on the alveolar phase of COVID-19. Am J Physiol-Lung Cell Mol Physiol. 2020 Jun 3;319(1):L115-20. [ Links ]
31. Alon R, Sportiello M, Kozlovski S, Kumar A, Reilly EC, Zarbock A, et al. Leukocyte trafficking to the lungs and beyond: lessons from influenza for COVID-19. Nat Rev Immunol. 2021 Jan;21(1):49-64. [ Links ]
32. Delorey TM, Ziegler CGK, Heimberg G, Normand R, Yang Y, Segerstolpe Å, et al. COVID-19 tissue atlases reveal SARS-CoV-2 pathology and cellular targets. Nature. 2021 Apr 29. [ Links ]
33. Ferreira AC, Soares VC, de Azevedo-Quintanilha IG, Dias S da SG, Fintelman Rodrigues N, Sacramento CQ, et al. SARS-CoV-2 engages inflammasome and pyroptosis in human primary monocytes. Cell Death Discov. 2021 Mar 1;7(1):1-12. [ Links ]
34. Peiris JSM, Yuen KY, Osterhaus ADME, Stöhr K. The Severe Acute Respiratory Syndrome. N Engl J Med. 2003 Dec 18;349(25):2431-41. [ Links ]
35. Erdinc B, Sahni S, Gotlieb V. Hematological manifestations and complications of COVID-19. Adv Clin Exp Med Off Organ Wroclaw Med Univ. 2021 Jan;30(1):101-7. [ Links ]
36. Terpos E, Ntanasis-Stathopoulos I, Elalamy I, Kastritis E, Sergentanis TN, Politou M, et al. Hematological findings and complications of COVID-19. Am J Hematol. 2020;95(7):834-47. [ Links ]
37. Tavares C de AM, Avelino-Silva TJ, Benard G, Cardozo FAM, Fernandes JR, Girardi ACC, et al. Alterações da ECA2 e Fatores de Risco para Gravidade da COVID-19 em Pacientes com Idade Avançada. Arq Bras Cardiol. 2020 Oct 13;115(4):701-7. [ Links ]
38. Tsitsiklis A, Zha B, Byrne A, DeVoe C, Levan S, Rackaityte E, et al. Impaired immune signaling and changes in the lung microbiome precede secondary bacterial pneumonia in COVID-19. Res Sq. 2021 Apr 23;rs.3.rs-380803. [ Links ]
39. Estimation of total mortality due to COVID-19. 2021. Disponible en:: http://www.healthdata.org/special-analysis/estimation-excess-mortality-due-covid-19- and-scalars-reported-covid-19-deaths [ Links ]
40. GUIAD-COVID-19. Reporte 10: Casos graves, críticos y muertes entre infectados por SARS-CoV-2 por franja etaria. 2021.Disponible en:: https://GUIAD COVID.github.io/publication/nota10/ [ Links ]
41. Adu-Amankwaah J, Mprah R, Adekunle AO, Noah MLN, Adzika GK, Machuki JO, et al. The cardiovascular aspect of COVID-19. Ann Med. 2021 Jan 1;53(1):227-36. [ Links ]
42. Wichmann D, Sperhake J-P, Lütgehetmann M, Steurer S, Edler C, Heinemann A, et al. Autopsy Findings and Venous Thromboembolism in Patients With COVID-19. Ann Intern Med. Internet. 2020 May 6. cited 2021 May 30. Disponible en:: Disponible en:: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7240772/ [ Links ]
43. Agbuduwe C, Basu S. Haematological manifestations of COVID-19: From cytopenia to coagulopathy. Eur J Haematol. 2020;105(5):540-6. [ Links ]
44. Godoy LC, Goligher EC, Lawler PR, Slutsky AS, Zarychanski R. Anticipating and managing coagulopathy and thrombotic manifestations of severe COVID-19. CMAJ. 2020 Oct 5;192(40):E1156-61. [ Links ]
45. Mueller C, Giannitsis E, Jaffe AS, Huber K, Mair J, Cullen L, et al. Cardiovascular biomarkers in patients with COVID-19. Eur Heart J Acute Cardiovasc Care. 2021 Mar 1;10(3):310-9. [ Links ]
46. Lei Y, Zhang J, Schiavon CR, He M, Chen L, Shen H, et al. SARS-CoV-2 Spike Protein Impairs Endothelial Function via Downregulation of ACE 2. Circ Res. 2021 Apr 30;128(9):1323-6. [ Links ]
47. Blüher M. Obesity: global epidemiology and pathogenesis. Nat Rev Endocrinol. 2019 May;15(5):288-98. [ Links ]
48. Sanchis-Gomar F, Lavie CJ, Mehra MR, Henry BM, Lippi G. Obesity and Outcomes in COVID-19: When an Epidemic and Pandemic Collide. Mayo Clin Proc. 2020 Jul 1;95(7):1445-53. [ Links ]
49. Pisabarro R, Gutierrez M, Bermúdez C, Prendez D, Recalde A, Chaftare Y, et al. Segunda Encuesta Nacional de Sobrepeso y Obesidad (ENSO 2) adultos (18-65 años o más). Rev Med Urug. 2009 Mar;25:14-26. [ Links ]
50. Ibarra A. Prevalencia y características clínicas de pacientes diabéticos ingresados en un hospital general. Arch Med Interna. 2015 Jul;37:57-60. [ Links ]
51. Bornstein SR, Rubino F, Khunti K, Mingrone G, Hopkins D, Birkenfeld AL, et al. Practical recommendations for the management of diabetes in patients with COVID-19. Lancet Diabetes Endocrinol. 2020 Jun;8(6):546-50. [ Links ]
52. Wijnant SRA, Jacobs M, Eeckhoutte HPV, Lapauw B, Joos GF, Bracke KR, et al. Expression of ACE2, the SARS-CoV-2 Receptor, in Lung Tissue of Patients With Type 2 Diabetes. Diabetes. 2020 Dec 1;69(12):2691-9. [ Links ]
53. Rubino F, Amiel SA, Zimmet P, Alberti G, Bornstein S, Eckel RH, et al. New-Onset Diabetes in Covid-19. N Engl J Med. 2020 Aug 20;383(8):789-90. [ Links ]
54. Sathish T, Kapoor N, Cao Y, Tapp RJ, Zimmet P. Proportion of newly diagnosed diabetes in COVID-19 patients: A systematic review and meta-analysis. Diabetes Obes Metab. 2021 Mar;23(3):870-4. [ Links ]
55. Al-Aly Z, Xie Y, Bowe B. High-dimensional characterization of post-acute sequalae of COVID-19. Nature. 2021 Apr 22;1-8. [ Links ]
56. Scappaticcio L, Pitoia F, Esposito K, Piccardo A, Trimboli P. Impact of COVID-19 on the thyroid gland: an update. Rev Endocr Metab Disord. 2020 Nov 25;1-13. [ Links ]
57. Chen W, Tian Y, Li Z, Zhu J, Wei T, Lei J. Potential Interaction Between SARS-CoV-2 and Thyroid: A Review. Endocrinology. Internet. 2021 Mar 1. cited 2021 Apr 30; 162(bqab004). Disponible en: Disponible en: https://doi.org/10.1210/endocr/bqab004 [ Links ]
58. Galanopoulos M, Gkeros F, Doukatas A, Karianakis G, Pontas C, Tsoukalas N, et al. COVID-19 pandemic: Pathophysiology and manifestations from the gastrointestinal tract. World J Gastroenterol. 2020 Aug 21;26(31):4579-88. [ Links ]
59. Elmunzer BJ, Spitzer RL, Foster LD, Merchant AA, Howard EF, Patel VA, et al. Digestive Manifestations in Patients Hospitalized With Coronavirus Disease 2019. Clin Gastroenterol Hepatol 2021 Jul;19(7):1355- 1365.e4. [ Links ]
60. Guo M, Tao W, Flavell RA, Zhu S. Potential intestinal infection and faecal-oral transmission of SARS-CoV-2. Nat Rev Gastroenterol Hepatol. 2021 Apr;18(4):269-83. [ Links ]
61. Xiao F, Tang M, Zheng X, Liu Y, Li X, Shan H. Evidence for Gastrointestinal Infection of SARS-CoV-2. Gastroenterology. 2020 May 1;158(6):1831-1833.e3. [ Links ]
62. Giron LB, Dweep H, Yin X, Wang H, Damra M, Goldman AR, et al. Plasma Markers of Disrupted Gut Permeability in Severe COVID-19 Patients. Front Immunol. Internet. 2021 cited 2021 Jul 25. Disponible en: https://www.frontiersin.org/articles/10.3389/fimmu.2021.686240/full [ Links ]
63. Yeoh YK, Zuo T, Lui GC-Y, Zhang F, Liu Q, Li AY, et al. Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID 19. Gut. 2021 Apr;70(4):698-706. [ Links ]
64. de-Madaría E, Capurso G. COVID-19 and acute pancreatitis: examining the causality Nature Reviews Gastroenterology Hepatology. Internet. 2021 cited 2021 Jul 25. Disponible en: Disponible en: https://www.nature.com/articles/s41575-020-00389-y [ Links ]
65. Solomon T. Neurological infection with SARS-CoV-2 - the story so far. Nat Rev Neurol. 2021 Feb;17(2):65-6. [ Links ]
66. Khan AR, Farooqui MO, Jatoi NN, Jawaid S, Mahdi D, Khosa F. Neurological Manifestations of SARS-CoV-2. The Neurologist. 2020 Dec 30;26(1):15-9. [ Links ]
67. Innes N, Johnson IG, Al-Yaseen W, Harris R, Jones R, Kc S, et al. A Systematic Review of Droplet and Aerosol Generation in Dentistry. medRxiv 2020.08.28.20183475. (preprint) 2020. Disponible en: https://www.medrxiv.org/content/10.1101/2020.08.28.20183475v1 [ Links ]
68. Meleti M, Cassi D, Bueno L, Bologna-Molina R. COVID-19 diffusion and its impact on dental practice in distant countries with similar ethnic background. Oral Dis. 2021 Apr;27 Suppl 3:720-2. [ Links ]
69. Teo AKJ, Choudhury Y, Tan IB, Cher CY, Chew SH, Wan ZY, et al. Saliva is more sensitive than nasopharyngeal or nasal swabs for diagnosis of asymptomatic and mild COVID-19 infection. Sci Rep. 2021 Feb 4;11(1):3134. [ Links ]
70. Eshraghi AA, Mirsaeidi M, Davies C, Telischi FF, Chaudhari N, Mittal R. Potential Mechanisms for COVID-19 Induced Anosmia and Dysgeusia. Front Physiology 2020 (Internet). (cited 2021 Apr 29). Disponible en: Disponible en: https://www.frontiersin.org/articles/10.3389/fphys.2020.01039/full [ Links ]
71. Sunavala-Dossabhoy G. Renin-angiotensin II-aldosterone axis in SARS-CoV-2- associated xerostomia. Oral Dis. 2020 Aug 7. [ Links ]
72. Marouf N, Cai W, Said KN, Daas H, Diab H, Chinta VR, et al. Association between periodontitis and severity of COVID-19 infection: A case-control study. J Clin Periodontol. 2021;48(4):483-91. [ Links ]
73. Ansari R, Gheitani M, Heidari F, Heidari F. Oral cavity lesions as a manifestation of the novel virus (COVID-19). Oral Dis. 2021 Apr;27 Suppl 3:771-2. [ Links ]
74. Brandini DA, Takamiya AS, Thakkar P, Schaller S, Rahat R, Naqvi AR. Covid-19 and oral diseases: Crosstalk, synergy or association? Rev Med Virol (Internet). (cited 2021 May 30). Disponible en:: https://onlinelibrary.wiley.com/doi/abs/10.1002/rmv.2226 [ Links ]
75. Martín Carreras-Presas C, Amaro Sánchez J, López-Sánchez AF, Jané-Salas E, Somacarrera Pérez ML. Oral vesiculobullous lesions associated with SARS-CoV-2 infection. Oral Dis. 2021 Apr;27 Suppl 3:710-2. [ Links ]
76. Nuno-Gonzalez A, Martin-Carrillo P, Magaletsky K, Martin Rios MD, Herranz Mañas C, Artigas Almazan J, et al. Prevalence of mucocutaneous manifestations in 666 patients with COVID-19 in a field hospital in Spain: oral and palmoplantar findings. Br J Dermatol. 2021 Jan;184(1):184-5. [ Links ]
77. Iebba V, Zanotta N, Campisciano G, Zerbato V, Di Bella S, Cason C, et al. Profiling of oral microbiota and cytokines in COVID-19 patients. bioRxiv 1;2020.12.13.422589. (preprint). 2020 Jan. Disponible en: https://www.biorxiv.org/content/10.1101/2020.12.13.422589v1 [ Links ]
78. Patel J, Sampson V. The role of oral bacteria in COVID-19. Lancet Microbe. 2020 Jul;1(3):e105. [ Links ]
79. Nalbandian A, Sehgal K, Gupta A, Madhavan MV, McGroder C, Stevens JS, et al. Post acute COVID-19 syndrome. Nat Med. 2021 Apr;27(4):601-15. [ Links ]
80. Wu Q, Zhou L, Sun X, Yan Z, Hu C, Wu J, et al. Altered Lipid Metabolism in Recovered SARS Patients Twelve Years after Infection. Sci Rep. 2017 Aug 22;7(1):9110. [ Links ]
81. Gou W, Fu Y, Yue L, Chen G, Cai X, Shuai M, et al. Gut microbiota may underlie the predisposition of healthy individuals to COVID-19. medRxiv 1;2020.04.22.20076091. (Preprint) 2020. Disponible en: https://www.medrxiv.org/content/10.1101/2020.04.22.20076091v1 [ Links ]
82. Chen Y, Gu S, Chen Y, Lu H, Shi D, Guo J, et al. Six-month follow-up of gut microbiota richness in patients with COVID-19. Gut (Internet). 2021 Apr 7 (cited 2021 May 1) Disponible en: Disponible en: https://gut.bmj.com/content/early/2021/04/07/gutjnl-2021-324090 [ Links ]
83. Ward DV, Bhattarai S, Rojas-Correa M, Purkayastha A, Holler D, Da Qu M, et al.The intestinal and oral microbiomes are robust predictors of covid-19 severity the main predictor of covid-19-related fatality. medRxiv 2021.01.05.20249061. (preprint). 2021 Jan 1. Disponible en: https://www.medrxiv.org/content/10.1101/2021.01.05.20249061v1 [ Links ]
84. Mukhtar K, Qassim S, Al Qahtani SA, Danjuma MI-M, Mohamedali M, Farhan HA, et al. A randomized trial on the regular use of potent mouthwash in COVID-19 treatment. medRxiv 2020.11.27.20234997. (preprint). 2021 Jan 1; Disponible en: https://www.medrxiv.org/content/10.1101/2020.11.27.20234997v2 [ Links ]
85. Institute for Health Metrics and Evaluation. COVID-19 has caused 6.9 million deaths globally, more than double what official reports show. 2021. Disponible en:: http://www.healthdata.org/news-release/covid-19-has-caused-69-million-deaths globally-more-double-what-official-reports-show [ Links ]
86. World Health Organization. Severe Acute Respiratory Syndrome (SARS). Disponible en: https://www.who.int/westernpacific/health-topics/severe-acute-respiratory-syndrome [ Links ]
87. World Health Organization. Middle East respiratory syndrome coronavirus (MERS-CoV). Disponible en: https://www.who.int/westernpacific/health-topics/middle-east-respiratory-syndrome coronavirus-mers [ Links ]
88. Wilder-Smith A. COVID-19 in comparison with other emerging viral diseases: risk of geographic spread via travel. Trop Dis Travel Med Vaccines. 2021 Jan 31;7(1):3. [ Links ]
89. Liu J, Li Y, Liu Q, Yao Q, Wang X, Zhang H, et al. SARS-CoV-2 cell tropism and multiorgan infection. Cell Discov. 2021 Mar 23;7(1):1-4. [ Links ]
90. Alene M, Yismaw L, Assemie MA, Ketema DB, Mengist B, Kassie B, et al. Magnitude of asymptomatic COVID-19 cases throughout the course of infection: A systematic review and meta-analysis. PLOS ONE. 2021 Mar 23;16(3):e0249090. [ Links ]
91. Oran DP, Topol EJ. Prevalence of Asymptomatic SARS-CoV-2 Infection: A Narrative Review. Ann Intern Med. 2020 Sep 1;173(5):362-7. [ Links ]
92. Johansson MA, Quandelacy TM, Kada S, Prasad PV, Steele M, Brooks JT, et al. SARS-CoV-2 Transmission From People Without COVID-19 Symptoms. JAMA Netw Open. 2021 Jan 7;4(1):e2035057. [ Links ]
93. Mir D, Rego N, Resende PC, Tort F, Fernández-Calero T, Noya V, et al. Recurrent Dissemination of SARS-CoV-2 Through the Uruguayan-Brazilian Border. Front Microbiol (Internet). 2021 (cited 2021 Jun 4). 12. Disponible en: Disponible en: https://www.frontiersin.org/articles/10.3389/fmicb.2021.653986/full [ Links ]
94. Elizondo V, Harkins GW, Mabvakure B, Smidt S, Zappile P, Marier C, et al. SARS-CoV 2 genomic characterization and clinical manifestation of the COVID-19 outbreak in Uruguay. Emerg Microbes Infect. 2021 Dec;10(1):51-65. [ Links ]
95. Salazar C, Díaz-Viraqué F, Pereira-Gómez M, Ferrés I, Moreno P, Moratorio G, et al. Multiple introductions, regional spread and local differentiation during the first week of COVID-19 epidemic in Montevideo, Uruguay. bioRxiv 2020.05.09.086223. (preprint). 2020 May 10; Disponible en: https://www.biorxiv.org/content/10.1101/2020.05.09.086223v1.full [ Links ]
96. Salazar C, Costabile A, Ferrés I, Perbolianachis P, Pereira-Gómez M, Simón D, et al. Case Report: Early Transcontinental Import of SARS-CoV-2 Variant of Concern 202012/01 (B.1.1.7) From Europe to Uruguay. Front Virol (Internet). 2021 (cited 2021 Jun). Disponible en: Disponible en: https://www.frontiersin.org/articles/10.3389/fviro.2021.685618/full [ Links ]
97. Cheng VCC, Lau SKP, Woo PCY, Yuen KY. Severe acute respiratory syndrome coronavirus as an agent of emerging and reemerging infection. Clin Microbiol Rev. 2007 Oct;20(4):660-94. [ Links ]
98. Shi W, Gao GF. Emerging H5N8 avian influenza viruses. Science. 2021 May 21;372(6544):784-6. [ Links ]
99. Pittaluga L, Deana A. Evidence-Based Policies in Uruguay Are Successful for Tackling COVID-19. Open J Polit Sci. 2020 Dec 2;11(1):21-33. [ Links ]
100. Trougakos IP, Stamatelopoulos K, Terpos E, Tsitsilonis OE, Aivalioti E, Paraskevis D, et al. Insights to SARS-CoV-2 life cycle, pathophysiology, and rationalized treatments that target COVID-19 clinical complications. J Biomed Sci. 2021 Jan 12;28(1):9. [ Links ]
Conflict of interest declaration: The authors have no conflict of interest regarding the publication of this paper.
Authorship contribution 1. Conception and design of study 2. Acquisition of data 3. Data analysis 4. Discussion of results 5. Drafting of the manuscript 6. Approval of the final version of the manuscript BM has contributed in 1, 2, 3, 4, 5, 6. AS has contributed in 5 and 6. BGF has contributed in 5 and 6. VPP has contributed in 1.2, 3, 4.5, 6.
Received: August 18, 2021; Accepted: September 14, 2021










 texto em
texto em