Serviços Personalizados
Journal
Artigo
Links relacionados
Compartilhar
Odontoestomatología
versão impressa ISSN 0797-0374versão On-line ISSN 1688-9339
Odontoestomatología vol.20 no.31 Montevideo jun. 2018
https://doi.org/10.22592/ode2018n31a2
Update
Influence of cannabis use on periodontal disease: a scoping review
1 Undergraduate Student, School of Dentistry, Universidad de la República. Montevideo, Uruguay.
2 Physiology Department, School of Dentistry, Universidad de la República. Montevideo, Uruguay
3 Postgraduate Degree in Dentistry, Universidad Federal de Pelotas, Brazil
4 Department of Dental Materials, School of Dentistry, Universidad de la República. Montevideo, Uruguay
5 Periodontics Department, School of Dentistry, Universidad de la República. Montevideo, Uruguay
Recent publications suggest the possible association between cannabis (Cannabis sativa) and periodontitis.
Objective
: To analyze the possible influence of cannabis use on periodontal disease, highlighting the available evidence and identifying the associated variables in the studies.
Materials and methods
: We conducted a scoping review applying a structured search method in PubMed/MEDLINE, Science Direct, LILACS, SciELO including publications until May 2017.
Results
: In vivo studies showed greater bone loss in animals exposed to cannabis. Clinical cases show that chronic cannabis use may result in gingival enlargement (with clinical features similar to phenytoin induced enlargement) and localized severe chronic periodontitis. Most of the epidemiological studies confirmed the possible association between cannabis and periodontitis.
Conclusions
: The consumption of cannabis seems to be associated with a greater presence of periodontitis. However, the specific mechanism by which cannabis acts in the gingival tissues is unknown.
Keywords: cannabis; periodontal diseases
Recientes publicaciones plantean la posible asociación del cannabis (Cannabis sativa) con el desarrollo de la periodontitis.
Objetivo:
Analizar la influencia del consumo de cannabis en la enfermedad periodontal, relevando la evidencia disponible e identificando las variables asociadas en los estudios .
Material y método:
se realizó una Scoping Review a partir de una búsqueda estructurada en PubMed/ MEDLINE, Science Direct, LILACS, SciELO hasta mayo de 2017 . Resultados: Estudios “In vivo” mostraron una mayor pérdida ósea en animales expuestos a Cannabis. Los casos clínicos muestran que el uso crónico de cannabis puede resultar en agrandamientos gingivales y periodontitis crónica severa localizada. Los estudios epidemiológicos demuestran una asociación estadística entre Cannabis y periodontitis.
Conclusiones:
El consumo del Cannabis parece estar asociado con una mayor presencia de periodontitis. Sin embargo, se desconoce el mecanismo específico por el cual actúa en los tejidos gingivales.
Palabras clave: cannabis; enfermedades periodontales
Introduction
Periodontitis is one of the most common chronic diseases, with a high prevalence that varies according to each population group1,2. It affects approximately 46% of adults in the United States of America1. In turn, a recent global burden report showed that over 11% of the world’s population presents severe forms of the disease2. In Latin America, almost 10% of the young population is affected by periodontal disease, and approximately 35% of children have gingivitis, with the highest prevalence rates in Colombia (77%) and Bolivia (73%)3. In adults, periodontitis affects up to 62.6% of individuals4. In Uruguay, data from the First National Survey of Oral Health showed that among young people (15 to 24), the percentage of individuals with no signs of bleeding, with absence of tartar and probing depth (PD) > 4mm was close to 30%5. Similarly, individuals aged between 35 and 44 had a 16.5% prevalence of moderate and 5.9% prevalence of severe periodontal disease respectively6. The highest prevalence was found in individuals aged between 65 and 74, where 34.7% had moderate periodontitis and 17% severe periodontitis6. These results emphasize the importance of prevention and treatment in oral health programs7.
Periodontal disease is mainly characterized by gingival inflammation, formation of periodontal pockets and destruction of the supporting tissues (alveolar bone and periodontal ligament)8. It is the result of the interaction of microbial biofilm (necessary etiological factor but insufficient in itself), a susceptible host and modulating factors9. Several studies have shown the existence of risk factors, among them tobacco10, diabetes11-14, obesity/overweight15,16 and genetic factors17,18. These elements modulate the host’s susceptibility or resistance with each microbial challenge9.
Recent publications suggest a possible association between cannabis (Cannabis sativa) and periodontitis. However, the use of cannabis components could have positive effects as they might reduce inflammatory processes23. If we consider the increase in the prevalence of cannabis use throughout the world (16% in the United States, 11% in France and 9% in Uruguay)24, it is essential to research the possible effects of cannabis on the oral cavity and periodontal tissues in order to understand its role in the onset of periodontal disease and to develop and lead appropriate public health policies.
Therefore, the aim of this scoping review is to analyze the possible influence of cannabis use on periodontal disease, surveying the available evidence and identifying the associated variables in the studies.
Methodology
Study design: The scoping review involves a systematic search but does not imply an analysis of the methodological quality of the studies. This review presents a summary of the articles available in the literature by providing an overview of the existing content, setting future research paths and pointing to the gaps in the literature25,26.
Search strategy: We conducted a scoping review applying a structured search method in PubMed/MEDLINE, Science Direct, LILACS, and SciELO including publications until May 2017. We used keywords and controlled terminology (MeSH terms) based on questions structured according to the PICO Model: “What is the possible influence of cannabis use on periodontal disease?” where we defined:
Population: individuals with periodontal disease.
Intervention: cannabis use
Control: individuals who do not use cannabis
Result: Occurrence or aggravation of periodontal disease
In this way, the following search strategy was implemented: (periodontal disease OR periodontitis OR gingivitis OR gingival disease) AND (cannabis OR marijuana). All study designs on humans and animals were included.
Study selection and eligibility criteria: Four researchers participated in the paper search (MM, AF, LC and EA) for the review design, and were advised by a librarian. The records were entered into EndNote (Thomson Reuters, Rochester, New York, NY, USA) to eliminate duplicates and create a virtual library. The researchers read and filtered the titles and abstracts for all records that complied with the predefined criteria. No language or year restriction was applied. All original papers were included. Letters to the editor, in vitro articles and reviews were not included in this review. Additionally, the references of each paper were traced in order to broaden the search.
Results
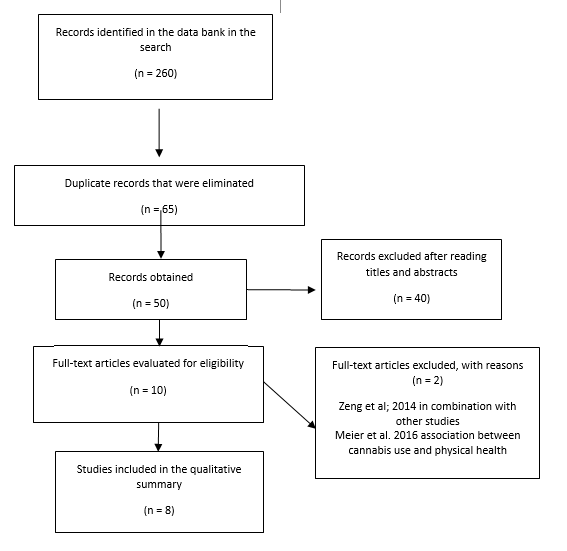
A total of 260 records were obtained from the search, of which 65 articles remained after removing duplicates. After reading titles and abstracts, 10 articles were selected to read the full text. Two of these articles were excluded27,28. The reasons for exclusion are presented in Figure 1. Finally, eight articles met the inclusion criteria and were included in this review (Table 1).
In vivo studies: Studies on mice evaluated the influence of the inhalation of cannabis and cannabidiol (CBD) on periodontal disease measuring attachment loss and bone density. Increased bone loss and lower bone density were observed in mice exposed to cannabis or cannabidiol, which shows that these substances can negatively influence periodontal tissues.
Clinical cases: Two clinical cases were included in this review29,30. These articles show that long-term cannabis consumption may lead to gingival enlargement with clinical features similar to the ones caused by phenytoin,30 in addition to chronic localized severe periodontitis29.
Epidemiological studies: four epidemiological studies were retrieved. Table 1 shows the main features of the studies. The methodological design of most studies was cross-sectional19,20,31, and there was a prospective study from a cohort of births27. Fifty percent of the studies were population-based19,27, while one was conducted with high school students20 and another with aboriginal communities from Australia. Three of the four studies were conducted on adults19,27,31, while only one evaluated adolescents20. Similarly, Shariff et al. 19 included patients with diabetes and previous periodontal treatment, while Lopez et al. 20 were the only ones that evaluated the patients’ oral hygiene habits.
Although cannabis exposure and the classification of periodontal disease have been categorized differently in the studies reviewed, cannabis use is linked to periodontal disease (Table 2). We observed a significant association between cannabis use and prevalence of periodontitis21,31, where cannabis users had a prevalence of periodontal disease 44% higher than that of non-users31. Similarly, population data from the United States National Health and Nutrition Examination Survey showed that the recreational use of cannabis was associated with advanced probing depth and clinical attachment loss19. Additionally, a study conducted on Chilean students found a Necrotizing Ulcerative Gingivitis (NUG) odds ratio that was 53% lower in individuals who had never used cannabis compared to frequent users20. The other associations were not statistically significant20.
Influence of cannabis on periodontal disease: Chart 1 shows the impact of cannabis on periodontal disease according to the action of its chemical components. Authors state that the causes of chronic inflammation in patients that use cannabis are the high temperatures and chemicals released during the consumption, followed by the clinical symptoms of xerostomy (as it has parasympatholytic properties), which would enhance the pathological effect32. We must remember that cannabis components that are not cannabinoids (products of combustion) are similar to those in tobacco and can have local and systemic effects 21,22,33. Only low CBD concentrations can have an anti-inflammatory effect, while high doses would have the opposite effect34.
Table 1: Chart comparing the epidemiological studies conducted to date. Relevant variables are identified to create a standardized research protocol to compare results
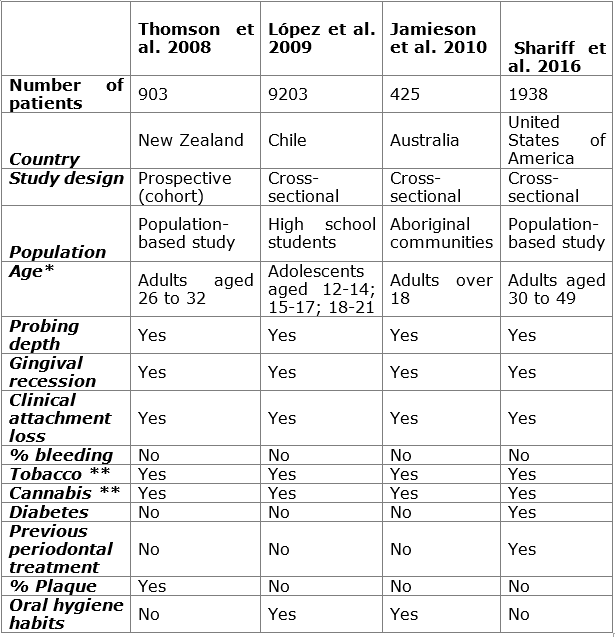
*Over 18 years of age. **Frequency, amount, and time elapsed since start of consumption, recreational or medical use.
Table 2: Summary of clinical studies aiming to prove the association between presence of periodontal disease and cannabis consumption
| Author | Cannabis exposure | Periodontal record | Periodontal disease |
|---|---|---|---|
| Association / Quality of the study | |||
| Thomson 2008 | “No exposure”, “Moderate exposure”, “High exposure” | 3 sites / 2 quadrants (26 years old) - Full mouth (32 years old) (except 3rd molars and implants) | CAL ≥ 3mm |
| Strong association between cannabis use and prevalence of periodontitis | |||
| López 2009 | “Never used cannabis”, “Regular use of cannabis” | 6 sites/Incisors, 1st and 2nd molars | CAL ≥ 3mm, NUG. |
| An association was found only when cannabis had never been used and with a lower NUG prevalence. There was no association for any other case | |||
| Jamieson et al. 2010 | “Never used or used only once” “Occasional use” “Frequent use” | 2 sites/Full mouth (except 3rd molars and implants) | PD ≥ 5mm and CAL ≥ 4mm (moderate periodontitis) |
| Cannabis users had a 44% higher prevalence of periodontal disease that individuals who do not use cannabis. | |||
| Shariff 2016 | “Never used” (Non-FRC) “Used every month for a year” (FRC) | 6 sites/Full mouth (except 3rd molars and implants) | PD ≥ 4mm and CAL ≥ 3mm (incipient periodontitis) |
| A strong association was found for FRC and presence of PD and CAL compared to non-FRC users. | |||
CCL: Clinical crown lengthening; PD: Probing depth; CAL: Clinical attachment loss; NUG: Necrotizing Ulcerative Gingivitis; FRC: Frequent recreational cannabis. Non-FRC: Non-frequent recreational cannabis.
Discussion
This work is the first scoping review that analyzes the connection between periodontal disease and cannabis consumption as it summarizes the evidence available. Eight articles were included, ranging from animal studies and clinical cases to observational (prospective and cross-cutting) studies, and indicating an influence and possible association of individuals who use cannabis and periodontitis. As this is a very important public health issue, health services should raise awareness of the risk that regular and occasional cannabis users run of having this pathology.
Cannabis sativa L. (hemp), has been known for many years for the use of its fibers in the textile industry, for clothing, material constructions and paper35,36. It was only in 1930 that isolated compounds called cannabinoids were isolated from cannabis. The most abundant ones within the extract are: Delta-9-tetrahydrocannabinol (THC), which has the strongest psychoactive effect, its precursor, cannabidiol, as well as cannabiol, a catabolic THC product formed spontaneously. These last two lack THC’s psychoactive effect, but have been found to have anticonvulsant properties35,37.
Currently, there are different types of cannabinoids according to their origin: 1) phytocannabinoids, derived from the cannabis plant; 2) endogenous cannabinoids (endocannabinoids), produced by the body itself of humans or other animals and 3) synthetic cannabinoids, with an identical chemical composition, but produced in the laboratory38. The characterization of these and other derivatives, as well as the receptors they interact with, have improved our understanding of the Endocannabinoid System35,39.
THC is the major psychoactive constituent and participates as a partial agonist for cannabinoid receptor type I (CB1) and type II (CB2). CBD is not psychoactive and is an antagonist for CB1 and CB2. It acts in multiple other receptors and may be an agonist for some systems39. The pharmacological effects of CBD are mediated by G-protein-coupled receptors, CB1 and CB2. When activated, CB1 receptors inhibit sympathetic transmission by acting on voltage-gated calcium and potassium channels, which are known to modulate epileptiform activity and seizures. CB2 receptors are primarily expressed in the immune system and have limited expression in the central nervous system. The effects of CBD are CB2 receptor-independent40,41. Thus, cannabis is usually smoked, vaped or consumed orally in the form of foods, teas or capsules41.
In this way, approximately 3.8% of the world’s population consumed cannabis in 2014 (183 million people): Iceland has the highest figure (18.3% of the population over the last 12 months), followed by the United States (16.2%), Chile in the fifth position (11.83%), France in the sixth (11.1%), and Uruguay coming in eleventh with 9.3%42. According to the World Drug Report 2011 (annual United Nations Office publication), in 2006, 14.8% of young people in Uruguay had consumed cannabis in the previous year, while only 6% of adults had used it43. The Final Report of the 5th National Household Survey on Drug Use of 2011 published by the National Drug Board in 201644 shows that since 2011 Uruguay has had an increase of three percentage points in marijuana consumption. This is the most widely used substance in the population after alcohol, tobacco and sedatives. Twenty-three percent of the people between the ages of 15 and 65 have used marijuana some time in their lives, and 9.3% say they used it in the last 12 months (161,000 people), and 6.5% in the last 30 days. There are 21,355 daily marijuana users in Uruguay.
Although cannabis is considered a “soft” drug which is only as harmful as coffee or tobacco, cannabis use can cause chronic secondary effects, such as periodontal disease21,32. The temperature of the combustion smoke is higher than that of cigarettes30. The lesions reported are similar to those of tobacco users, but always less severe30. Both acute and chronic consumption lead to nicotine stomatitis and uvulitis30. This connection between tobacco and cannabis is even closer: a study reports that not all tobacco users smoke cannabis, but 90% of cannabis consumers smoke tobacco45. This makes it difficult to independently diagnose the effects of both types of substances in the studies. This is why some reports conduct statistical analyses also with individuals who do not smoke tobacco, and the association with periodontal pathologies remains19. As most individuals who smoke cannabis also use tobacco31, this may enhance the harmful effects of both drugs.
Multiple studies show the association of cannabis with high levels of oral biofilm, dental caries, candida albicans (but not candidiasis)41, leukoedema, leukoplakia46 and stomatitis37,41. In addition, excessive exposure to cannabis was associated with an increase in respiratory diseases and in the prevalence of cancer of the oropharynx39. Reports indicate that 58.3% of women and 47.4% of men reported pulpitis during cannabis consumption. Pulpitis could be added to the list of adverse vascular effects associated with cannabis use already reported (conjunctivitis, tachycardia, hypotension, angina pectoris)47,48. Thus, it is important to note that only low concentrations of CBD can have an anti-inflammatory effect and that high doses are harmful34. In the case of oral cancer, proinflammatory and carcinogenic compounds have been found in cannabis smoke, similar to the composition found in tobacco smoke, including carbon monoxide22,33,49,50.
THC and CBD stimulate the release of prostaglandin E2 (PGE2) from synovial cells and inhibit the in vitro synthesis of leukotrienes of human nuclear polymorphic cells51. Additionally, it has also been reported that CBDs suppress proinflammatory mediators52-54 such as IFNγ (gamma interferon), FNT-α (tumor necrosis factor-alpha), IL-1b (interleukin 1 beta)55 and IL-10 (interleukin 10)56. In another study, where CBD doses were systemically injected in rats, researchers concluded that they are an emerging class of mediators that might participate in the control of periodontal pathologies as they help reduce inflammation57. Contrary to these findings, other authors conducted the same clinical trial, noting increased bone loss in the furcation area of the teeth with induced periodontitis. However, no effect was noted on periodontally healthy sites, which could be related to an alteration of the immune function during the bone resorption process, or even the activation of specific receptors that could increase bone destruction58. In this sense, there has been some speculation regarding the endocannabinoid system, which could play a role in the regulation of bone metabolism59,60.
In addition, we identified specific receptors for this substance in periodontal tissues, which was followed by an increase in the release of anandamide, an agonist for the cannabinoid receptor derived from arachidonic acid61. Other studies confirm these data: the relationship between the endocannabinoid system (receptors and mediators produced by our body) and periodontal disease was studied, and researchers detected a proliferation of gingival fibroblasts when the system was activated, suggesting a new path for periodontal disease therapy62,63. A recent study shows how human gingival mesenchymal stem cells that were pre-treated with CBD before the transplant increase their survival rate in the host as they modulate their immune and inflammatory response41,64.
In turn, in 2012 Rawal et al.63 described two clinical pictures in patients and consumers. The features that always appeared were inflammation of papillae and gingival margin, with presence of nodular areas, similar to those in patients that consume dilantin (phenytoin), mainly in anterior teeth. Their analysis is very interesting as they find possible coincidences in these clinical pictures, finding similarities in the chemical components of both substances: 1) cannabidiol (CBD) is also used as an anticonvulsant, 2) CBD may increase the effects of phenytoin and phenobarbital, 3) CBD and phenytoin have a similar structure, displaying rings in the same pattern. The authors conclude that both comply with the stoichiometric requirements suggested for the action of an anticonvulsant, deducing that inflammation may be caused by the same mechanism63.
In 2016, Momen-Heravi et al. reported the case of a 23-year-old female patient diagnosed with periodontal disease. The patient reported daily use of cannabis for three years. The author recounts inflammation of papillae and gingival margins, mainly at the anterior region of the mandible, where the cannabis cigarette was placed. The X-ray showed loss of alveolar bone in that area29. Regarding the treatment, the authors recommend behavior modification and non-surgical and surgical therapy for the successful management of cannabis-related periodontitis29.
In this context, the first epidemiological study that analyzed the relationship between cannabis use and periodontal disease was conducted in 2008 by Thomson et al.21 in New Zealand. The population was made up of adults born in a hospital in the country in 1972 and 1973. The periodontal clinical examination was performed on two occasions, at ages 26 and 32. The recording system included two quadrants on three sites per tooth, examining Gingival Recession (GR), Probing Depth (PD) and Clinical Attachment Loss (CAL). Cannabis exposure was assessed applying a self-report methodology. From this information, three groups were obtained: 1) no exposure 2) low exposure (1-40 times in the previous year) and 3) high exposure (over 41 exposures in the previous year). The study also enquired about socioeconomic status, tobacco use, sex, reason for attending the dental clinic, and biofilm accumulation21.
Of the patients, 32.3% had not consumed cannabis in the previous year, and 47.4% and 20.2% had done so at low and high exposure rates respectively. Most consumers were men, of low socioeconomic status and who rarely sought dental care, with significant levels of bacterial plaque21. This confirms the previous findings that show these individuals tend not to worry about their health, which in turn can be linked to oral pathologies32. Of the patients, 33% were tobacco smokers and 17% ex-smokers. This is consistent with Fairman45 in that frequent cannabis consumers were also tobacco users, and as age increased, tobacco consumption increased too32.
The reported results show that cannabis use was strongly associated with the prevalence of periodontitis, with the greatest differences found in PD > 5mm. However, no association was found between the consumption of both substances at the same time and periodontal disease. According to Thomson, the use of cannabis may be a risk factor for periodontal disease when used independently from tobacco, and as age increases so do prevalence and incidence21.
The association with Necrotizing Ulcerative Gingivitis (NUG) was also researched on a sample of adolescents. In this case, six sites were measured and observed in the incisors and second molars, and the NUG diagnosis was made20. Like Thomson et al., they found that it was a limitation not to know the dose and length of cannabis use, as the questionnaire included “never consumed” or “regular consumption” with 18.9% and 6% respectively. Of the regular consumers, only 16.3% were smokers, contrary to what Thomson et al. and Fairman found. The NUG diagnosis did not consider pain or bleeding, hence its high prevalence compared to other studies; no association with cannabis consumption was found20,21,45.
In turn, in 2016, Shariff observed the same in adults in the United States, where 26.6% were frequent cannabis users. From that group, 29.2% had PD ≥ 4mm, 24.8% PD ≥ 6 mm and 24.5% PD ≥ 8mm, comparing these data to that of non-frequent users: 22.3%, 19.2% and 18.9%. They concluded that frequent cannabis use is associated with increased probing depth and gingival recession, as well as higher likelihood of severe periodontitis19.
Although the studies have varying methodologies, in vivo studies, clinical cases and epidemiological studies seem to indicate an association between cannabis use and periodontal disease; this may increase bone loss, exacerbating or initiating periodontitis. These authors recommend including the variables there presented (Table 1) to achieve a standardized research protocol, non-existent so far, to be able to compare the results of different studies conducted in the future. The authors recommend that further epidemiological studies, preferably prospective or case-control studies, be conducted, since clinical trials would be ethically unacceptable. In addition, it is important that future research include statistical analyses, checking if individuals are tobacco users in order to reduce any potential bias. The results of this review should be interpreted with caution since they are based on studies that apply very different methodologies.
Conclusions
The specific mechanism by which cannabis acts on gingival tissues is unknown due to the insufficient number of studies conducted so far and the differences in methodologies and in the populations studied. However, cannabis consumption seems to make periodontal disease worse. This is why health services should take action to raise awareness of the strong likelihood of regular cannabis users having this pathology
Referencias
1. Eke PI, Dye BA, Wei L, Slade GD, Thornton-Evans GO, Borgnakke WS, et al. Update on Prevalence of Periodontitis in Adults in the United States: NHANES 2009 to 2012. J Periodontol. 2015; 86 (5): 611-22. [ Links ]
2. Kassebaum NJ, Bernabé E, Dahiya M, Bhandari B, Murray CJL, Marcenes W. Global Burden of Severe Periodontitis in 1990-2010. J Dent Res. 2014; 93 (11): 1045-53. [ Links ]
3. Botero JE, Rösing CK, Duque A, Jaramillo A, Contreras A. Periodontal disease in children and adolescents of Latin America. Periodontol 2000. 2015; 67 (1): 34-57. [ Links ]
4. Oppermann R V., Haas AN, Rösing CK, Susin C. Epidemiology of periodontal diseases in adults from Latin America. Periodontol 2000. 2015; 67 (1): 13-33. [ Links ]
5. Lorenzo S, Piccardo V, Alvarez F, Massa F, Alvarez R. Enfermedad Periodontal en la población joven y adulta uruguaya del Interior del país. Relevamiento Nacional 2010-2011. Odontoestomatologia. 2013; 15 (Noespecial): 35-46. [ Links ]
6. Lorenzo SM, Alvarez R, Andrade E, Piccardo V, Francia A, Massa F, Correa MB, Peres MB. Periodontal conditions and associated factors among adults and the elderly?: findings from the first National Oral Health Survey in Uruguay. Cad Saúde Pública. 2015; 31 (11): 2425-36. [ Links ]
7. Albandar JM, Goldstein H. Multi-Level Statistica Models in Studies of Periodontal Diseases. Am Acad Periodontol. 1992; 63 (8): 690-5. [ Links ]
8. Kim J, Amar S. Periodontal disease and systemic conditions: a bidirectional relationship. Odontology. 2006; 94 (1): 10-21. [ Links ]
9. Rioboo Crespo M, Bascones a. Factores de riesgo de la enfermedad periodontal: factores genéticos. Av en Periodoncia e Implantol Oral. 2005; 17 (2): 69-77. [ Links ]
10. Johannsen A, Susin C, Gustafsson A. Smoking and inflammation: Evidence for a synergistic role in chronic disease. Periodontol 2000. 2014; 64 (1): 111-26. [ Links ]
11. Pihlstrom BL. Periodontal risk assessment, diagnosis and treatment planning. Periodontology 2000. 2001; 25: 37-58. [ Links ]
12. Mealey BL, Oates TW. Diabetes Mellitus and Periodontal Diseases. J Periodontol. 2006; 77 (8): 1289-303. [ Links ]
13. Khader YS, Dauod AS, El-Qaderi SS, Alkafajei A, Batayha WQ. Periodontal status of diabetics compared with nondiabetics: A meta-analysis. J Diabetes Complications. 2006; 20 (1): 59-68. [ Links ]
14. Nelson RG, Shlossman M, Budding LM, Pettitt DJ, Saad MF, Genco RJ, Knowler WC. Periodontal disease and NIDDM in Pima Indians. Diabetes Care. 1990;13 (8): 836-40. [ Links ]
15. Keller A, Rohde JF, Raymond K, Heitmann BL. Association between periodontal disease and overweight and obesity: a systematic review. J Periodontol. 2015;86 (6): 766-76. [ Links ]
16. Chaffee BW, Weston SJ. Association Between Chronic Periodontal Disease and Obesity: A Systematic Review and Meta-Analysis. J Periodontol. 2011; 81 (12): 1708-24. [ Links ]
17. Negrato CA, Tarzia O, Jovanovic L, Chinellato LEM. Periodontal disease and diabetes mellitus. J Appl Oral Sci. 2013; 21 (1): 1-12. [ Links ]
18. Preshaw PM, Alba AL, Herrera D, Jepsen S, Konstantinidis A, Makrilakis K, Taylor R. Periodontitis and diabetes: a two-way relationship. Diabetologia. 2012; 6; 55 (1):21-31 [ Links ]
19. Shariff JA, Ahluwalia KP, Papapanou PN. Relationship Between Frequent Recreational Cannabis (Marijuana and Hashish) Use and Periodontitis in Adults in the United States: National Health and Nutrition Examination Survey 2011 to 2012. J Periodontol. 2016; 88 (3) :273-80. [ Links ]
20. López R, Baelum V. Cannabis use and destructive periodontal diseases among adolescents. J Clin Periodontol. 2009; 36 (3): 185-9. [ Links ]
21. Thomson WM, Poulton R, Broadbent JM, Terrie E, Caspi A, Beck JD, et al. Cannabis Smoking and Periodontal Disease Among Young Adults. Jama. 2008; 299 (5): 525-31. [ Links ]
22. Ashton CH. Pharmacology and effects of cannabis?: a brief review. Br J psychiatry. 2001; 178:101-6. [ Links ]
23. Naftali T, Mechulam R, Lev LB, Konikoff FM. Cannabis for inflammatory bowel disease. Dig Dis. 2014; 32 (4): 468-74. [ Links ]
24. Biehl JR, Burnham EL. Cannabis smoking in 2015: A concern for lung health? Chest. 2015;148 (3): 596-606. [ Links ]
25. Arksey H, O'Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005; 8 (1): 19-32. [ Links ]
26. Conde MCM, Chisini LA, Sarkis-Onofre R, Schuch HS, Nör JE, Demarco FF. A scoping review of root canal revascularization: relevant aspects for clinical success and tissue formation. Int Endod J. 2017; 50 (9): 860-74. [ Links ]
27. Meier MH, Caspi A, Cerdá M, Hancox RJ, Harrington H, Houts R, et al. Associations Between Cannabis Use and Physical Health Problems in Early Midlife. JAMA Psychiatry. 2016; 73 (7): 731. [ Links ]
28. Zeng J, Williams SM, Fletcher DJ, Cameron CM, Broadbent JM, Shearer DM, et al. Reexamining the Association Between Smoking and Periodontitis in the Dunedin Study With an Enhanced Analytical Approach. J Periodontol. 2014; 85 (10): 1390-7. [ Links ]
29. Momen-Heravi F, Kang P. Management of cannabis-induced periodontitis via resective surgical therapy: A clinical report. J Am Dent Assoc. 2016; 148 (3): 179-84. [ Links ]
30. Rawal SY, Dabbous MK, Tipton DA. Effect of cannabidiol on human gingival fibroblast extracellular matrix metabolism: MMP production and activity, and production of fibronectin and transforming growth factor ß. J Periodontal Res. 2012; 47 (3): 320-9. [ Links ]
31. Jamieson LM, Gunthorpe W, Cairney SJ, Sayers SM, Roberts-Thomson KF, Slade GD. Substance use and periodontal disease among Australian Aboriginal young adults. Addiction. 2010;105 (4): 719-26. [ Links ]
32. Versteeg PA, Slot DE, van der Velden U, van der Weijden GA. Effect of cannabis usage on the oral environment: a review. Int J Dent Hyg. 2008;6 (4): 315-20. [ Links ]
33. Taylor DR, Hall W. Respiratory health effects of cannabis: position statement of the Thoracic Society of Australia and New Zealand. Intern Med J. 2003; 33: 310-3. [ Links ]
34. Berdyshev E V. Cannabinoid receptors and the regulation of immune response. Chem Phys Lipids. 2000;108 (1-2): 169-90. [ Links ]
35. Kalant H. Medical use of cannabis: history and current status. Pain Res Manag. 2001; 6 (2) :80-91. [ Links ]
36. Lehmann T, Sager F, Brenneisen R. Excretion of cannabinoids in urine after ingestion of cannabis seed oil. J Anal Toxicol. 1997; 21 (5): 373-5. [ Links ]
37. Maloney W. Significance of cannabis use to dental practice. New York State Dent Assoc. 2011;77 (3): 36-9. [ Links ]
38. Ali EMM, Almagboul AZI, Khogali SME, Gergeir UMA. Antimicrobial Activity of Cannabis sativa L . Chin Med. 2012; 3: 61-4. [ Links ]
39. Murnion B. Medicinal cannabis. Aust Prescr. 2015; 38 (6): 212-5. [ Links ]
40. Welty TE, Luebke A, Gidal BE. Cannabidiol: Promise and Pitfalls. Epilepsy Curr. 2014;14 (5): 250-2. [ Links ]
41. Greydanus DE, Hawver EK, Greydanus MM, Merrick J. Marijuana: Current Concepts. Front Public Heal. 2013;1: 1-17. [ Links ]
42. UNODC. Informe Mundial sobre las Drogas. publicación las Nac Unidas. Viena: UNODC 2016. [ Links ]
43. UNODC. Informe Mundial sobre las Drogas. Publicación las Nac Unidas, núm Vent S11XI10. 2011 [ Links ]
44. Uruguay. Junta Nacional de Drogas. VI Encuesta Nacional en Hogares sobre Consumo de Drogas, 2016: Informe de Investigacion. Montevideo: Junta Nacional de Drogas, 2016 [ Links ]
45. Fairman BJ. Cannabis Problem Experiences Among Users of the Tobacco- Cannabis Combination Known As Blunts. Drug Alcohol Depend. 2015; 150:77-84. [ Links ]
46. Kayal RA, Elias WY, Alharthi KJ, Demyati AK, Mandurah JM. Illicit drug abuse affects periodontal health status. Saudi Med J. 2014; 35 (7): 724-8. [ Links ]
47. Shekarchizadeh H, Khami M, Mohebbi S, Ekhtiari H, Virtanen J. Oral Health of Drug Abusers?: A Review of Health Effects and. Iran J Publ Heal. 2013; 42 (9): 929-40. [ Links ]
48. Madnier I. Illicit drugs for toothache: Letter to editor of Brit Dent J. Br Dent J. 2002; 192( 3): 120-1. [ Links ]
49. Benson MK, Bentley AM. Editorials Lung disease induced by drug addiction. Thorax. 1995; 50: 1125-7. [ Links ]
50. WU T-C, Tashkin DP, Djahed B, Rose JE. Pulmonary Hazards of Smoking Marijuana as Compared with Tobacco. N Engl J Med. 1988; 318 (6): 347-51. [ Links ]
51. Phillipson JD. 50 Years of Medicinal Plant Research - Every Progress in Methodology is a Progress in Science. Planta Med. 2003; 69 (6): 491-5. [ Links ]
52. Melamede R. Cannabis and tobacco smoke are not equally carcinogenic. Harm Reduct J. 2005; 2: 21. [ Links ]
53. Galve Roperh I, Sanchez C, Cortes ML, Gomez del Pulgar T, Izquierdo M, Guzman M. Anti-tumoral action of cannabinoids: involvement of sustained ceramide accumulation and extracellular signal-regulated kinase activation. Nat Med. 2000; 6: 313-9. [ Links ]
54. Heeschen C, Jang JJ, Weis M, Pathak A, Kaji S, Hu RS, et al. Nicotine stimulates angiogenesis and promotes tumor growth and atherosclerosis. Nat Med. 2001;7 (7): 833-9. [ Links ]
55. Watzl B, Scuderi P, Watson RR. Influence of marijuana components (THC and CBD) on human mononuclear cell cytokine secretion in vitro. AdvExpMedBiol. 1991;288: 63-70. [ Links ]
56. Sacerdote P, Martucci C, Vaccani A, Bariselli F, Panerai AE, Colombo A, Parolaro D, Massi P. The nonpsychoactive component of marijuana cannabidiol modulates chemotaxis and IL-10 and IL-12 production of murine macrophages both in vivo and in vitro. J Neuroimmunol. 2005;159(1-2):97-105. [ Links ]
57. Napimoga MH, Benatti BB, Lima FO, Alves PM, Campos AC, Pena-dos-Santos DR, Severino FP, Cunha FQ, Guimarães FS. Cannabidiol decreases bone resorption by inhibiting RANK/RANKL expression and pro-inflammatory cytokines during experimental periodontitis in rats. Int Immunopharmacol. 2009; 9 (2): 216-22. [ Links ]
58. Nogueira-Filho GR, Todescan S, Shah A, Rosa BT, Tunes U da R, Cesar Neto JB. Impact of Cannabis Sativa (Marijuana) Smoke on Alveolar Bone Loss: A Histometric Study in Rats. J Periodontol. 2011;82 (11): 1602-7. [ Links ]
59. Idris AI, Sophocleous A, Landao-Bassonga E, Van't Hof RJ, Ralston SH. Regulation of bone mass, osteoclast function, and ovariectomy-induced bone loss by the type 2 cannabinoid receptor. Endocrinology. 2008; 149 (11): 5619-26. [ Links ]
60. Idris AI, Van't Hof RJ, Greig IR, A RS, Al. E. Regulation of bone mass, bone loss and osteoclast activity by cannabinoid receptors. 2005; 11 (7): 774-9. [ Links ]
61. Nakajima Y, Furuichi Y, Biswas KK, Hashiguchi T, Kawahara KI, Yamaji K, et al. Endocannabinoid, anandamide in gingival tissue regulates the periodontal inflammation through NF-?B pathway inhibition. FEBS Lett. 2006; 580 (2): 613-9. [ Links ]
62. Kozono S, Matsuyama T, Biwasa KK, Kawahara K ichi, Nakajima Y, Yoshimoto T, Yonamine Y, Kadomatsu H, Tancharoen S, Hashiguchi T, Noguchi K, Maruyama I. Involvement of the endocannabinoid system in periodontal healing. Biochem Biophys Res Commun. 2010; 394 (4): 928-33. [ Links ]
63. Rawal SY, Tatakis DN, Tipton DA. Periodontal and Oral Manifestations of Mariiuana Use. J Tenn Dent Assoc. 2012; 92 (2): 26-31. [ Links ]
64. Libro R, Scionti D, Diomede F, Marchisio M, Grassi G, Pollastro F, et al. Cannabidiol Modulates the Immunophenotype and Inhibits the Activation of the Inflammasome in Human Gingival Mesenchymal Stem Cells. Front Physiol. 2016; 7: 1-17. [ Links ]
Received: October 17, 2017; Accepted: March 21, 2018










 texto em
texto em