Alfalfa-nodulating rhizobia
The rhizobia that fix nitrogen in legumes of the genus Medicago belong to two closely related species, Ensifer meliloti and Ensifer medicae (syn. Sinorhizobium meliloti and Sinorhizobium medicae, respectively). While both rhizobia species displayed a nitrogen-fixing phenotype with the model Medicago truncatula, E. meliloti establishes symbiosis with Medicago sativa, M. littoralis, and M. tornata, annual alfalfa that grows naturally in neutral to alkaline pH soils, E. medicae is associated with M. polymorpha, M. arabica and M. murex, annual legumes adapted to moderately acid soils1)(2. Biondi and others3 suggested a preferential relationship between E. meliloti and tetraploid Medicago spp., and between E. medicae and diploid species such as many annual medics. Based on a genotypic and biochemical characterization, Garau and others1 proposed that E. meliloti and E. medicae were adapted to different species of the genus Medicago according to the niches that these legumes occupy in their natural habitat, although more studies are necessary to confirm this association.
In addition, the characterization of the populations of alfalfa-nodulating rhizobia from acid soils showed the presence of another lineage of Rhizobium sp. that forms ineffective nodules in alfalfa. This group of poorly characterized rhizobia known generically as the Oregon type was initially represented by the Or191 strain4. These rhizobia were isolated from M. sativa nodules in Oregon (1981-82) from a field having moderately acid soil conditions (pH 5.5 to 5.7), where alfalfa had not been cultivated for at least 10 years. Unlike E. meliloti and E. medicae, the rhizobia strains of this group generated small colonies, did not acidify and did not grow at 39 °C in YEM4. This type of persistent, highly competitive, and inefficient rhizobia in alfalfa was also identified in acid and moderately acid soils from various locations in United State of America (USA), Australia and Canada4.
In Argentina and Uruguay, populations of alfalfa-nodulating rizhiobia from acid soils were also characterized5. A collection of 466 strains were studied and distributed in two groups, the main group consisted of efficient nitrogen-fixing rhizobia and a minor group of inefficient and acid-tolerant rhizobia formed by isolates similar to strain Or191. The sensitivity of E. meliloti to acidity was observed to be in the range of pH between 5.6 and 6.06, depending on the strain and the level of Ca+2 in the culture medium. Conversely, the group of the inefficient alfalfa-nodulating rhizobia grew at pH 5.0 and showed similar phenotypic characteristics among all inefficient isolates, as well as similar to those of strain Or191. For example, these inefficient strains, unlike to E. meliloti and E. medicae, showed inability to grow in LB media at 28 °C and TY media at 37 °C, shared the same plasmid patterns, lipopolysacharide profiles, insertion-sequence fingerprints and ERIC, MBOREP1 and BOXC1PCR-fingerprinting patterns, nodulated Phaseolus vulgaris and Leucaena leucocephala7, and different species of the Trigonella and Melilotus genera8. These characteristics were shared with the strain Or191 isolated from acid soils of Oregon, USA. Some phenotypic, genotypic and symbiotic characteristics of alfalfa-nodulating rhizobia are summarized in Figure 1.
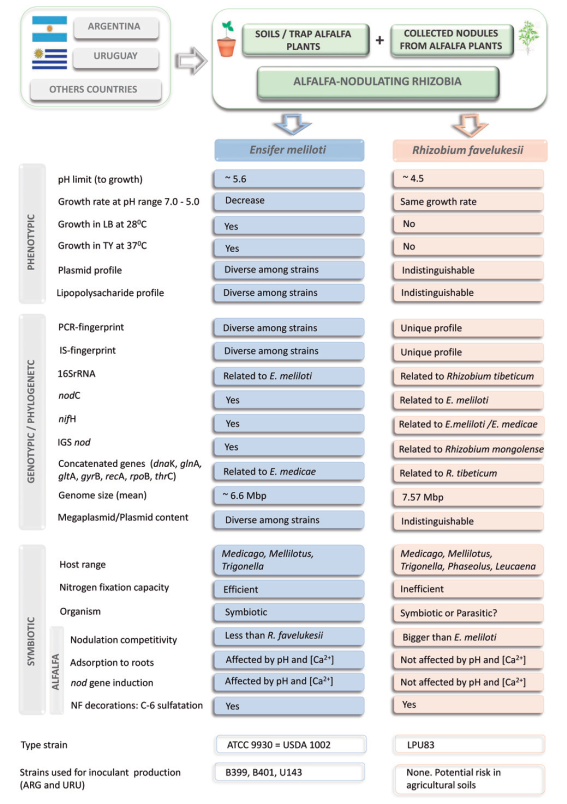
Figure 1: Main phenotypic, genotypic and symbiotic characteristics of alfalfa-nodulating rhizobia isolated from Argentina and Uruguay. References: IS,insertion sequences; IGS, intergenic sequences; NF, Nod Factor.
The genetic analysis of the symbiotically inefficient rhizobia demonstrated a very homogeneous genetic background among all isolates and strain Or1918. Among the inefficient strains characterized by Del Papa and others5, and later by Wegener and others8, the LPU83 strain isolated from Argentina was selected as a representative strain of the acid-tolerant alfalfa-nodulating rhizobia. The LPU83 strain, for which the genome sequence is available9, and Rhizobium sp. Or191 belongs to a novel species named R. favelukesii. The type strain of this species is LPU8310.
Three out of five isolated strains from Uruguayan soils characterized by Del Papa and others5 corresponded to E. meliloti, and were identified in soils from Colonia, Paysandú, and Soriano. The remaining two strains, CE20 and CE26, were collected in soils from Colonia with pH 5.9 and, like Or191 and LPU83 strains, were able to growth in LB and TY media at 28 °C and 37 °C respectively5. Thus, the initial characterization of CE20 and CE26 strains indicated that R. favelukesii strains are present in Uruguayan soils5)(11.
Although R. favelukesii strains are considered a potential risk in acid soils in which alfalfa is cultivated12, Del Papa and others5 observed only a low proportion of nodules occupied by them. However, in acid soils they are highly competitive for the nodulation of alfalfa13, thus, its presence could explain the ineffective nodulation of alfalfa in acid soils around the world4)(13. In this regard, in a field experiment in Ontario, Canada, M. sativa was grown at a single site that had no known history of alfalfa cultivation for two seasons in slightly acid field soil (pH 6.1), and a predominant group of phage-resistant bacteria was isolated from the nodules14. From those isolates recovered from alfalfa, 69% presented a single genotype that was indistinguishable from strain Or191 by the genetic analysis employed15. Consequently, this finding indicates that: i) the genetic uniformity among the R. favelukesii isolates is independent of their geographical origin, and ii) soil persistence of E. meliloti, and the acid-tolerant R. favelukesii isolates in the presence/absence of alfalfa plants is not the same under soil acid stress conditions16.
When phylogenetic studies for elucidating the genomic relationship of the R. favelukesii were performed, the 16S rRNA gene sequences of the R. favelukesii LPU83t and Or191 strains were found to be identical (100%), while respective identities of 99.9 and 99.2% were found with Rhizobium tibeticum CCBAU 85039T and Rhizobium grahamii CCGE 502t10. However, different results were obtained from the phylogenetic evaluation of symbiotic genes. For instance, the analysis of the nodC gene, encoding the N-acetylglucosaminyltransferase that catalyzes the first reaction in the synthesis of the Nod factor core17, indicated that the E. meliloti nodC gene is the most closely related one and suggested that both nodC genes were originated from a common ancestor18.
A distinctive feature of the rhizobia-alfalfa symbiosis is the marked plant-bacteria specificity and the strict requirement for Nod factors sulfated at its reducing end. In this context, the presence of a functional nodH-encoded NF sulfotransferase in R. favelukesii LPU83 was reported16, and phylogenetic analyses based in this gene also pointed to the close relationship of this group with the alfalfa-nodulating rhizobia found in previous studies18. The nifH genetic tree furthermore demonstrated that R. favelukesii strains form a new clade, but the group was also closely related to the tight phylogenetic cluster formed by E. meliloti and E. medicae. Also, the nod cluster of R. favelukesii LPU83 has a marked synteny with the clusters of E. meliloti and E. medicae, but the intergenic region between nodE and nodG, which has a characteristic length of Medicago-nodulating rhizobia, is similar to Rhizobium mongolense strains12. Furthermore, a robust phylogenetic analysis involving concatenation of seven genes (dnaK, glnA, gltA, gyrB, recA, rpoB, and thrC)19)(20 and applying the Maximum-Pairwise, Neighbor-Joining and Maximun-Likehood methods indicated that R. favelukesii LPU83 is located close to a clade where Rhizobium leguminosarum and Rhizobium etli were situated, as had been previously observed for other housekeeping genes.
Restrictions on rhizobia-alfalfa symbiosis imposed by acid pH and Al3+
The problems of implantation and establishment of rhizobia-alfalfa symbiosis are usually due to the acidic pH of the soil or to high concentrations of available aluminum (Al3+), higher than 3 mg.kg-1 soil21. Both factors negatively affect the rhizobial growth and survival22)(23)(24)(25)(26)(27 and interfere with the legume-rhizobia symbiosis by affecting rhizobial attachment to roots and the nod gene expression28)(29.
In acid soils, the production of cultivated legumes is lower than in neutral soils, due to the factors that independently or in combination affect: i) the host plant28)(30, ii) the rhizobia population31, and iii) the interaction between the two5)(32)(33)(34. It was estimated that approximately 25%of the world’s soils are acidic or are going through a process of acidification35, and among the nodule-inducing bacteria, E. meliloti is the most sensitive to acid pH34)(36. Because of this, alfalfa infection by its specific rhizobia is reduced in acid and moderately acid soils2)(4.The initial stages of symbiosis are susceptible to pH and Al3+ because they negatively affect rhizobia binding to root cells and nod gene expression28)(29)(37. Arora and others38 demonstrated that while RMP5 strain of E. meliloti is more tolerant to metal stress than Bradyrhizobium sp. strain BMP1, high concentrations of Al3+ affected bacterial growth, nitrogenase, nitrate reductase and nitrite reductase activities. Moreover, the growth rate of E. meliloti was shown to be lower at acid pH, although this could be improved in the presence of millimolar calcium concentrations28. Soto and others29, studied the effect of pH and Ca2+ on diverse aspects of alfalfa nodulation with E. meliloti and the acid-tolerant and inefficient R. favelukesii LPU83, and observed that the addition of 6 mM Ca2+ at pH 5.6 increased the number of nodules per plant elicited by E. meliloti 2011 but not by R. favelukesii LPU83. Unlike E. meliloti, the attachment of the acid-tolerant R. favelukesii LPU83 to alfalfa roots is not greatly affected by pH or Ca2+ concentration. In addition, media acidification weakens nod gene induction in E. meliloti strains but not in R. favelukesii LPU83. Moreover, the addition of Ca2+ at low pH does not affect either nod gene expression in alfalfa-nodulating rhizobia (R. favelukesii or E. meliloti) nor equality of nod gene inducers exuded by alfalfa plants. Therefore, Soto and others29 suggested that, in divergence to other symbiotic systems, the most limiting factor in the establishment of the E. meliloti-alfalfa symbiosis at low pH is the attachment of bacterial cells to plant roots.
Consequently, any approach to improve the symbiotic performance of E. meliloti in acid soils must be focused on solving the rhizobial attachment to alfalfa roots at low pH. Concerning R. favelukesii, neither the root binding nor the nod gene expression was affected by acid pH or Ca2+, indicating that the genetic background of R. favelukesii LPU83 may be useful for improving the performance of E. meliloti in acid pH soils.
In addition to the microsymbiont, plants are also negatively affected by acid pH and high Al3+ concentrations21)(28. At toxic concentrations, Al3+ inhibits root growth and therefore decreases the absorption of nutrients by plants. In alfalfa, it was shown that Al3+ inhibits the synthesis of indol acetic acid (IAA) in apical buds and its transport, and stimulates the synthesis of callose that prevents symplastic translocation. This leads to an imbalance in the distribution of IAA in the roots, responsible for their defective growth39.
The low pH and a high content of soluble Al3+ in soils disturb several physiological and biochemical processes, including nitrogen fixation, which significantly reduces the productivity and quality of alfalfa under field conditions40. Although conventional and transgenic varieties of alfalfa with variable grades of stress tolerance were developed41)(42, none of them were shown to reach optimum levels of nitrogen content or give good forage yield under low pH and high Al3+ conditions. This fact can be partially attributed to the inability of these stress-tolerant germplasms to preserve the beneficial plant-microbe interactions under stressful environments. While there is a robust legal framework for the regulation of transgenic plants and there are hundreds of commercial transgenic crops worldwide released for commercial agriculture production, practically no country allows the release of genetically modified microorganisms into agricultural ecosystems, and so, there is no genetically modified acid pH-resistant rhizobia inoculant in the market. In this context, commercial transgenic plants should be associated with beneficial microorganisms isolated from nature and free of genetic manipulations, at least in the near future.
The development of rhizobial inoculants is discussed below in the text, and in terms of alfalfa cultivars suitable in low pH - higth Al3+ soils have not been generated yet21.
Response of alfalfa to inoculation
The presence of efficient rhizobia populations in the soils where legumes are cultivated hinders the observation of the response to inoculation. In Uruguay, this phenomenon has been observed for clovers43)(44 and Lotus45, but not alfalfa, for which the practice of inoculation represents a clear advantage46. Studies carried out in different regions of Europe showed a high native-naturalized microbial diversity that efficiently nodulate alfalfa34)(47)(48)(49, probably the result of the history of this crop introduced about 3,000 years ago50. In Spain, it is common to cultivate alfalfa without inoculation, although farmers from the North of the peninsula have sporadically used the strain GRO15 (= ISM-16; conversation with Rodríguez-Navarro; unreferenced), supplied by IFAPA (ex INIA) Seville51. However, Ramírez-Bahena and others34 showed that under controlled conditions, inoculation of alfalfa with selected acid-tolerant strain improved plant biomass production. In Serbia, where the use of commercial inoculants for alfalfa is not a common practice52, Stajkoviæ-Srbinoviæ and others47 found that in soils with pH between 5.1 and 8.1 most of the nodules of M. sativa were occupied by E. meliloti. Additionally, Deliæ and others52 identified effective native alfalfa strains in acid soils, which represented an interesting finding because 50% of Serbia’s arable soils are acid. The effectiveness of these Serbian strains, present in soils with and without a history of alfalfa cultivation, was not influenced by the soil nor the host genotype52. In France, populations of rhizobia that nodulate alfalfa were identified in neutral soils (pH 6.8), even after 10 years without cultivating this legume53. In Germany, the presence of rhizobia in soils with pH 5.9 to 6.5 was undetectable after 8 years without alfalfa, but after inoculating with strain L33 and growing alfalfa, at least 48%of the nodules were occupied by native strains54. In soils from 10 sites of United Kingdom with moderately acid to alkaline soils (pH 5.8 to 8.2), Roberts and others48 showed that not all of them had strains of E. meliloti and in most of them the inoculation significantly increased the number of nodules and biomass production.
In Oceania and America, where alfalfa was introduced 300-500 years ago50, a clear response to inoculation is commonly observed46)(55)(56. For instance, in New Zealand alfalfa was observed to be highly dependent on the inoculation because it failed to grow in soils where alfalfa-nodulating rhizobia were absent or ineffective57. Likewise, in Australia, alfalfa inoculation is also necessary and extensive inoculant development was carried out as documented by Bullard and others58.
In soils from tropical areas of Brazil, Ferreira and others59 showed that there was no native population of E. meliloti in the soil and the use of two commercial inoculants, under controlled conditions, increased the nodulation and productivity of three alfalfa cultivars. Oliveira and others60 also observed a positive response to inoculation with the strain SEMIA-116, which make unnecessary the use of nitrogen fertilizers for alfalfa in the field.
In Argentina, Chile and Uruguay, different responses to inoculation, in relation to the pH and the population of E. meliloti present in the soil before cultivating, were observed by Racca and others46. In soils from Argentina with pH 6.2 with a high rhizobial population (5.8x103 rhizobia per gram of soil) a 13% higher yield was recorded in the treatment without inoculation than in the treatment inoculated with the commercial strain, indicating the presence of efficient naturalized populations. In contrast, a marked response to inoculation was observed in soils of Argentina and Uruguay with pH between 5.4 and 6.1, in which no rhizobia populations were detected, reaching increases of 109 % to 199% of biomass. Moreover, in Chilean soils with pH 5.7 and a moderate rhizobial population (1x103 rhizobia per gram of soil), a high response to inoculation was also observed (98% of biomass increase). Of note, the persistence of E. meliloti after harvesting the crop is low46)(61, particularly in acid soils, making the practice of inoculation in those cases necessary.
In addition to edaphic conditions, the alfalfa cultivar used can also determine a different response upon strain used as inoculant. In controlled conditions, Blair57 observed different symbiotic efficiency in cultivars with different origins (subsp. sativa or subsp. falcata), whereas in field conditions the author identified some effective strains in a wide range of cultivars. On the other hand, in soils of Brazil containing lime, Oliveira and others60 and Ferreira and others59 did not observe different responses of the alfalfa ‘Crioula’ non-dormant cultivar when inoculated with different strains. Nevertheless, Hartel and Bouton62 demonstrated that in acid soils the performance of alfalfa genotypes selected for acidity tolerance was enhanced by inoculant strains of rhizobia selected also for tolerance to acid pH. Of note, most alfalfa cultivars selected and used in the Southern Cone have not been accompanied by development rhizobia inoculants, or by studies considering the interaction cultivate alfalfa x rhizobia strain. In this sense, recommended inoculant strains should be evaluated with the commercial cultivars in the intended environment where they are going to be grown.
Liming has been a solution to improve alfalfa production in many countries with acid soils, such as in Brazil63, Chile64, Argentina65, whereas this practice has not become widespread in Uruguay66. In any case, liming has economic and practical restrictions21, so the development of inoculants that could establish efficient symbiosis with the cultivars used in acid soils is a strategy that must be strengthened.
Selection of rhizobia strains for development of alfalfa inoculants
Biological nitrogen fixation in agriculture can be improved with the use of rhizobial inoculants developed with strains selected by their high performance in target cultivars grown in specific soils and environments. Among the criteria for selecting strains as alfalfa inoculants, their tolerance to acid pH and persistence in acid soils should be considered, and in some cases their tolerance to Al3+(13)(16)(21)(29)(64. The tolerance to both acid pH and high Al3+ concentrations is rare, therefore these bacterial genotypes would be a minority within populations present in the soils67. Thus, developing a rhizobial inoculant suitable for such condition is a challenge, but the needing to promote a sustainable agriculture is leading several countries to make an effort towards this aim.
In Australia, a lot of work was done to select suitable inoculants for alfalfa and other legumes. For instance, different commercial inoculants were developed for perennial and annual Medicago species21)(30)(58 in order to increase the production in acid and alkaline soils1. Acid soils with high levels of Al+3 constitute a problem that affects alfalfa cultivation in large areas of Australia and also of New Zealand. In these countries, E. meliloti strain RRI128 is used as a commercial inoculant21. This strain, which establishes symbiosis with perennial and annual Medicago species, was isolated from a nodule from the roots of barrel medic (M. truncatula) grown in a greenhouse in Victoria soil of Australia68. However, its origin is unclear and it is believed that it was isolated for the first time in 1995 in New Zealand69. While the RRI128 strain has been used in Australia since 200058, Wigley and others21 recently showed that two strains evaluated at acidic conditions and at different concentrations of Al3+ were more effective than RRI128 in alfalfa, making them promising inoculants.
In Argentina, Chile and Uruguay the selection of rhizobia for alfalfa inoculants also focused on obtaining strains suitable for establishing efficient symbiosis in acid soils, and in some cases to high concentrations of Al3+. In Chile, alfalfa cultivation is an alternative for soils with pH 770)(71, and soils of the south with pH 5.5 and high content of Al3+, where strain AG-06 was identified as promising when evaluated in greenhouse conditions64. In Argentina, moderate acid-tolerant and efficient strains were obtained, among them the strain LPU63, which was evaluated under controlled conditions13)(16. In addition, an evaluation of strains from different cultivated areas, concluded with the recommendation of E. meliloti strain B399 (= R. meliloti 102F34), which is currently the used inoculant for alfalfa in Argentina72. This strain is, almost genetically, equal to strain 1026 of E. meliloti, although it has a different symbiotic phenotype73.
Regarding the inoculation of alfalfa in Uruguay, between 1964 and 1990 the strain U45 was used as a commercial inoculant74. This strain was isolated in Uruguay but its geographical origin is unclear. Between 1991 and 2003 an inoculant based on U137 and U143 strains was used, both isolated from Uruguayan soils, and since 2004 to date the strain U143, which is more stable than strain U4575, has been used as a commercial inoculant. The strain U143 is used in soils with a pH between 5.0 and 7.7 in the dairy region, where 63 % of soils have pH <6.0 and 37% pH <5.776. This level of acidity is critical for alfalfa nodulation and for the survival of E. meliloti. It should be noted that the intensification of milk production increased the use of short rotations based on grasses and nitrogen fertilizers77. This practice enhances the acidification of the soil.
The symbiotic efficiency of U143 strain, which does not persist in acid soils, was lower than the symbiotic efficiency of CE21, CE41 and CE47 strains under controlled conditions11. Currently, a project that aims to develop an inoculant for alfalfa suitable for soils in the dairy region of Uruguay (Faculty of Agronomy - INIA, 2018 - 2021) identified 3 strains, among 250, with symbiotic efficiency equal to or greater than the U143 strain, under controlled conditions at pH 6.5 and pH 5.6. Strains S8, E9 and L14 are promising for their efficiency at pH 5.6, which deserves further attention and evaluation under field conditions, as described in the following section.
Interestingly, in Australia ten different commercial inoculants for alfalfa have been used between 1953 and 2003, roughly one per decade58. This implies that inoculant selection should be seen as a continuous process, coupled to the selection and implementation of novel plant cultivars, as well as to changes in the cropping areas and in soil/environmental conditions. Moreover, the genetic stability of the bacterial strains should also be considered, because long-term storage could involve genotypic and phenotypic changes78. Bloem and others79 showed differences in the phenotypes of the U45 strain from successive agar subcultures and the lyophilized parental strain, stored for 15 years. Among the differences they found altered ability to fix nitrogen, which reinforces the necessity to check inoculants periodically and make passages through the host under field conditions80.
As mentioned above, acid tolerance was a major criterion to select for alfalfa inoculants. Additionally, the selection of strains that exhibit the so-called adaptive acid-tolerance response (ATR) must be considered. The ATR is defined as the resistance of cells to an acid shock when they have been previously grown at a moderately low pH. However, validation experiments with soil microcosms and on-field have not been carried out yet. It will be important to determine the possibility to preserve the physiology of acid-adapted rhizobia (ATR+) in inoculants formulations -based on ATR+ strains. Draghi and others81 demonstrated that the ATR+ can be induced in E. meliloti, as shown previously for E. medicae, and that the entrance of E. meliloti into the adaptive ATR occurs under batch cultivation conditions at moderately acid pH. In marked contrast, no increased tolerance to hydrogen ions was obtained if rhizobia are grown in a chemostat under continuous cultivation at same pH. In addition, they showed that ATR+ significantly increased (30%) the competitiveness for nodule occupancy at low pH. In E. medicae, the two-component sensor-regulator system, actSR, was shown to be important for the induction of the ATR+ phenotype82. In other organisms the ATR+ phenotype confers cross-resistance to other stresses as well, such as heat, ethanol, and sodium chloride83)(84.The basic aspects of the ATR+ phenotype have not been extensively characterized, and further research is needed to increase our knowledge on the bacterial mechanisms involved in this adaptive response. Clearly, the rational manipulation of the rhizobial ATR will require a detailed physiological and functional characterization of the processes. Meanwhile, the ATR phenotype can be an additional criterion to consider when selecting for acid-tolerant rhizobia.
Strategy for the development of alfalfa inoculants in Uruguay
The maximum potential of rhizobia-alfalfa symbiosis is not achieved at acid pH, so worldwide efforts are made in order to have suitable inoculants for that situation16)(27)(46)(52)(62)(85.
In Uruguay, the Biochemical and Microbiology Laboratories of the Faculty of Agronomy and the INIA Pastoral and Forage Improvement Group developed an efficient and competitive rhizobial inoculant for clover43)(44. The current aim is to develop an alfalfa inoculant following the strategy used for clover (Figure 2).
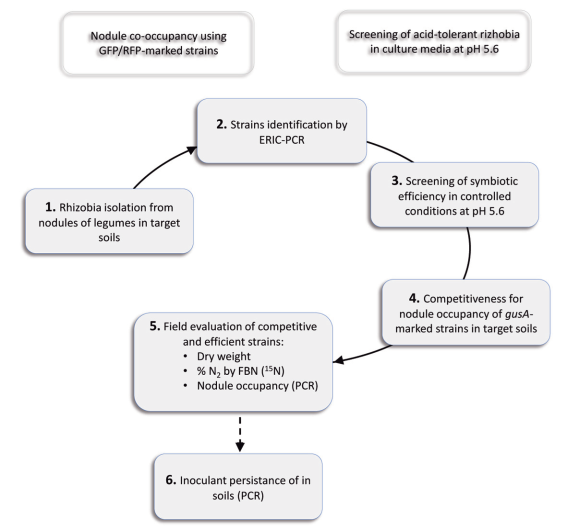
Figure 2: Scheme of the different stages followed for the selection of alfalfa inoculants in acid soils.
In this sense, 69 strains were identified by their ERIC profiles, among 250 isolates of plants grown in soils with pH between 5.3 and 6.0, from 9 different sites belonging to the location known as «Dairy Basin». Symbiotic efficiency of these strains, and others from available collections, was evaluated and compared with the commercial inoculant under controlled conditions in pots with vermiculite:sand. Based on this criterion, in the Chaná cultivar, 16 strains were pre-selected in trials at pH 6.5 according to the biomass production accumulated in two cuts (Figure 3). The strains were then evaluated in plant trials at pH 5.6 in sand with irrigation solution buffered with MES13. The promising strains in that situation were S8, E9 and L14 isolated from La Estanzuela, Colonia (pH between 5.5 and 6.0), and SJ2 isolated from Juan Soler, San José (pH 5.7). Additionally, to the criteria used in the selection of clover strains, it is considered appropriate to incorporate for the development of an alfalfa inoculant, the evaluation of the acid tolerance of the strains and the inoculant persistence in the field. Although acid tolerance in culture media is considered a favorable characteristic when selecting a rhizobia strain, this tolerance does not necessarily correlate with the persistence in acid soils or with the ability to express its symbiotic phenotype16. Evaluating the persistence of inoculants in the soils where it is used is interesting since the commercial inoculant currently used in Uruguay is not persistent, particularly when used in acid soils. As this characteristic is only known after several years of evaluation, the evaluation of persistence is included as the final stage of the selection strategy (Figure 2).
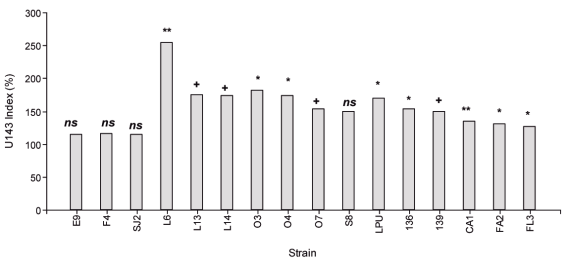
Figure 3: Cumulative production of aerial biomass in 45 days after cultivated under controlled conditions, expressed as % of strain U143. E9, L6, L13, L14, 03, 04, 07 and S8 strains isolated from Colonia, SJ2 from San José, and F4, FA2 and FL3 from Florida. LPU63 strains isolated in Argentina (Collection of the University of La Plata), U136 and U139 Uruguay (National Collection of strains from Ministry of Livestock, Agriculture and Fisheries). The data were analyzed using Mixed Generalized Linear Models (Gamma Family and Log link) InfoStat®86 and the comparison of means was performed using the post-hoc DGC test of each strain with U14387. The strains were higher than U143 at p <0.01 (**), p <0.05 (*), p <0.1 (+), ns corresponds to strains similar to U143. The N treatment (not shown) produced 109% more biomass than the commercial inoculant (p <0.001).
Although Uruguay has an outstanding position in the production and use of rhizobia inoculants, the commercial strains currently used for clovers, Lotus and alfalfa were selected about 50 years ago in agronomic environments that have changed. These changes are a consequence of the displacement of cultivated pastures to other sites, the type of planting (conventional tillage versus non-tillage) and the use of new cultivars. For this reason, it is advisable to consider development of rhizobia inoculants as a continuous process that improves the competitiveness and persistence of strains in soils. In the case of alfalfa in particular, this will contribute to increasing the stability of annual and summer forage production and will lead to an expansion of the cultivation area.