1. Introduction
In recent years, cannabis cultivation (Cannabis sativa L.) has spread throughout the vast majority of Uruguayan departments, with a greater influence in the south and east of the country1.
Being a relatively new crop in the country, it is important to know the health problems that can affect it. In this regard, pests and fungi associated with diseases in five cannabis crops were inspected during the production cycle in 2018 and 2019, in the south of the country. A total of 11 plants with symptoms of stem canker were observed in three inflorescence-production crops, from which Neofusicoccum sp.2 was isolated.
Neofusicoccum parvum ((Pennycook & Samuels) Crous, Slippers & A.J.L. Phillips 2006) along with other species in the Botryosphaeriaceae family cause canker and blight in several crops and woody species3. Moreover, the pathogen was first reported in cannabis crops in Italy4 and subsequently in the United States5. In Uruguay, N. parvum affects the crops of apple tree6)(7, pear, peach6 and vine8, but it is unknown whether it can cause the disease in cannabis. Thus, this study aims to characterize three isolates of Neofusicoccum by morphological and molecular analysis, and pathogenicity tests in cannabis plants.
2. Material and methods
2.1 Isolates of Neofusicoccum
Three of 11 purified isolates of Neofusicoccum sp. were used from the Phytopathology Laboratory collection (Agronomy College, Udelar). The isolates were obtained from plants with canker symptoms in stems collected during 2018 and 2019 (Table 1).
2.2 Morphological identification
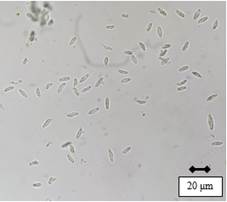
Each isolate was placed onto Petri dishes for morphological identification, with potato dextrose agar (PDA) medium (Oxoid Ltd., Hampshire, England) at 25 °C, and colony characteristics were observed at 3, 8 and 16 d incubation, using the description by Pennycook & Samuels9) and Phillips and others10. Furthermore, the isolates were incubated on water agar (WA) medium (Oxoid Ltd., Hampshire, England) with addition of sterile pine acycles, at 25 °C with 12 h photoperiod of UV-light for a period of 49 d, for the production of reproductive structures (pycnidia). Once the pycnidia were formed, the color, shape, and presence (or not) of conidial septa were observed with an Olympus CX23 optical microscope (China), magnification of 40x5)(11. In addition, the length and width of 30 conidia per isolate were recorded with the Dinno Capture 2.0 program.
2.3 Molecular identification
The colonies used were grown in PDA medium, incubated for five days at 25 °C in the dark, and the genomic DNA was extracted by Quick-DNA™ Fungal/Bacterial Mini-prep Kit (Zymo Research, USA), following the manufacturer's instructions. The DNA concentration of the samples was determined using a Nanodrop 2000 Thermo Scientific spectrophotometer, adjusting the concentration to 25 ng/μl. Amplification was performed by PCR with primers RPB2bot6F and RPB2bot7R from the partial region of the second major subunit of RNA polymerase II (RBP2)11. Amplification conditions consisted of an initial denaturation at 94 °C for 4 m, followed by 10 cycles at 94 °C for 20 s, at 58 °C for 48 s, and at 72 °C for 45 s, and 25 cycles at 94 °C for 20 s, 56 °C for 40 s and 72 °C for 45 s; lastly, a final extension of 10 m at 72 °C. PCR amplification was carried out in a Peltier PTC-100 thermocycler (USA). PCR products (500 bp) were confirmed on a 1.5% agarose gel and sent for sequencing to Macrogen Inc., Korea. The obtained sequences were edited and manually corrected using the MEGA program version 1012 and compared with deposited in GenBank through the BLAST search tool13.
2.4 Pathogenicity tests
The “Queen Dream” hemp variety was used for the pathogenicity test, an American variety obtained by Blue Forest Farm with a 0.22% THC content14.
The 30-day-old plants were transplanted into 0.8-liter pots with GrowMix® Multipro substrate. The three isolates grown in PDA for 5 d at 25 °C were used for inoculation (Table 1). Fifteen plants were inoculated per isolate, plus 12 plants for the non-inoculated control. The inoculation consisted of making a wound with a sterile toothpick, at the height of the cotyledons, and subsequent placement of a mycelium disk of 5 millimeters in diameter. Sterile PDA media discs were also placed in the control treatment plants. All discs were fixed to the wound with parafilm5 and the plants were covered for 48 h with plastic bags previously moistened with sterile distilled water. The incidence of the disease was evaluated from 7 to 14 days post-inoculation under conditions of 25 ºC and a photoperiod of 16 h.
2.5 Reisolation
Once the symptoms and signs were observed, the pathogen was reisolated from the inoculated plants. Three symptomatic plants were taken per treatment for this purpose; pycnidia were extracted from the wounds with the help of a disinfected dissection needle and placed in Petri dishes with PDA medium and incubated in the dark at 25 ºC. The obtained colonies and conidia were characterized by morphology and compared with the characteristics of the initial isolates.
3. Results
3.1 Morphological identification
Three days after incubation, a whitish aerial mycelium with a cottony texture was observed, with very faint yellow pigment production from the center, observable on the back of the plate (Figures 1a and 1b). At eight days of incubation, the colonies presented a dense aerial mycelium with white-grayish colors observable from the front and olive green on the reverse of the plate (Figures 1c and 1d). At 16 days, the colonies presented a compact dark-colored mycelium, ranging from gray to black on the front and olive green to black on the back (Figure 1e and 1f).
After 49 days of incubation, it was possible to observe the presence of pycnidia with abundant hyaline conidia with smooth edges and without septa, in shapes ranging from ellipsoidal to spindle-shaped (Figure 2). The conidia sizes (n=30) of Cluc F, Migues 1 and Migues 14 averaged 16.6 μm (14.1-19.7) × 8.3 μm (7.1-9.7), 17.1 μm (14.4-19.8) × 8.4 μm (7.1-9.5), and 17.2 μm (14.4-19.7) × 8.4 μm (7.3-9.8), respectively.
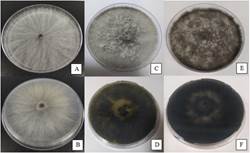
Figure 1: Isolate colony of N. parvum. on the front and back of the plate in PDA medium after 3 (A-B), 8 (C-D) and 16 (E-F) days of incubation
3.2 Molecular identification
The sequences Cluc F, Migues 1 and Migues 14 were deposited in the Gen-Bank (access number: OQ621792, OQ621793 and OQ621794, respectively). These showed 100% identity with N. parvum (MT592407.1) for the partial sequence of the second major subunit of RNA polymerase II.
3.3 Pathogenicity and reisolation test
Seven days after the inoculation, a brown darkening of the stem could be observed upwards from the inoculation area of the plant. As the disease progressed, it caused widespread wilting in the plant. At 14 days, black pycnidia developed on the stem wound (Figure 3). The incidence of disease was 86.6%, 73.3% and 60% in plants inoculated with Migues 14, Culc F and Migues 1, respectively. N. parvum was reisolated from symptomatic plants from stem pycnidia with characteristics consistent with the isolates used. Plants inoculated with PDA discs did not exhibit symptoms or signs of disease (Figure 3).
4. Discussion
The study consisted of identifying and determining the pathogenicity of three isolates of Neofusicoccum obtained from plants with symptoms of stem canker. The isolates exhibited the phenotypic characteristics of the colonies and conidia described by Phillips and others10 for Neofusicoccum spp.
During colony observation, it was possible to verify the production of yellow pigment, a recently observed characteristic in isolates of N. parvum according to a study carried out by Abdollahzadeh and others15.
Values obtained of conidial size were within those presented by Phillips and others10 for N. parvum 17.1 μm (12-24 μm) × 5.5 μm (4-10 μm), also similar to those of Delagado-Cerrone and others7, 18.61 ± 2.33 × 7.38 ± 1.07. As for the characteristics of the conidia, they coincide with the description of Phillips and others10, although no darkening of the conidia was observed over time, nor the presence of septa. These characteristics were not observed either in the isolates of Alberti and others4 and Delagado-Cerrone and others7, but may or may not occur within the species due to their high variability.
By analyzing the sequences of the partial region RBP2 N. parvum was identified. This region was also used to differentiate species from the complex N. parvum/N. ribis.11.
The results of the pathogenicity tests showed that the isolates caused disease symptoms2 in cannabis plants.
In Uruguay, N. parvum infects several plant species, but symptoms in cannabis had not been recorded. Thus, this study confirms N. parvum causing stem canker in cannabis, for the first time in Uruguay. Knowing the causal agent of the disease in hemp will allow the implementation of management strategies to reduce crop losses.