1. Introduction
The olive tree (Olea europaea L.) has been grown in the Mediterranean region for centuries. Its cultivation has been expanded to other regions of the world, mainly between latitude 30° and 45°; regions with hot and dry summer and cold and wet winter. In lower latitudes the cold necessary for bud differentiation is conferred by altitude, allowing its cultivation1. The species was introduced in Brazil by the Portuguese around 1800, arousing great interest in Rio Grande do Sul and Minas Gerais states. Experimental works and small plantations prior to 1979 are reported in Santa Catarina state. However, even demonstrating a high productive potential, the old plantations, maintained without adequate management techniques or the support of agricultural research in this field, were eliminated due to phytosanitary problems and gradually abandoned2.
Olive trees grow at relatively low temperatures. In Maria da Fé, Minas Gerais, it was estimated that the basal temperature is 10.5 to 11 ºC3. Differences are observed between varieties in the heat requirement for growth4, which affects their adaptation. The duration of the phenological stages depends on heat accumulation, measured in degree-days. The amount of heat (temperatures above 10 ºC) required since flowering to reach maturity is variable between varieties. For a high production of olive oil, it is ideal to accumulate 4,100 degree-days, despite in some regions maturation is reached with 1,300 degree-days5.
The process of flower induction in olive is not well known. The initial stimulus for bud floral differentiation has been believed to occur even in the summer, during its formation in the shoot tips, a six-week long process. Some factors can affect the induction: the presence of fruits with seeds in the shoot, bud and shoot position, radiation, nutritional status and water balance6)(7)(8. However, recent evidences of induction-related gene expression contest the summer induction theory, suggesting that it happens when trees are exposed to at least 75 days with temperatures reaching levels below 15 °C9.
The morphological changes in the buds until the winter rest are very subtle, practically imperceptible10. Winter temperature should ideally reach 2 to 18 ºC to promote the floral differentiation of the induced buds11. Yet, the need for chilling hours for good floral differentiation varies across cultivars. Without a minimum chilling flowering can even occur, but in low intensity12. In northwestern Argentina, Catamarca, La Rioja and Sumalao, at 28º latitude, similar to Santa Catarina, the occurrence of chilling is much lower than that observed in the Mediterranean region, and considered insufficient for most of the varieties13.
The State of Santa Catarina (southern Brazil) is a favorable place for olive cultivation when considering the maximum and minimum air temperature, but marginal in terms of rainfall, which is excessive14. Another mapping of the state with macro regional average bioclimatic indexes points out that the western region is unsuitable15. More recently, the western Santa Catarina region was classified as risky due to insufficient chilling and frost frequency16.
From 2008 to 2014, Koroneiki olive cultivar produced more than a thousand liters of oil per hectare per year in a trial in Santa Catarina (Caçador municipality, 1,033 m of elevation), but in Chapecó (638 m of elevation) the flowering and yield have been fickle through years17)(18. The causes of variation have not been established, but based on the literature16, we hypothesize insufficient chilling, and high air temperature and humidity at flowering are the most likely.
The objectives of this work were to characterize the reproductive phenology and quantify some productive indexes of 3 olive cultivars (Arbequina, Arbosana and Koroneiki) in western Santa Catarina warm, humid subtropical climate.
2. Materials and methods
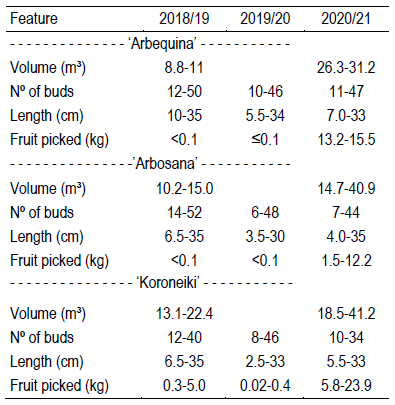
The study was performed in Chapecó, Santa Catarina, Brazil, at 650 m elevation and -27.1° of latitude. The climate is subtropical with warm summers (Cfa, Köppen), and the soil is a dystrophic Red Latosol. In March, 2018, 5-year-old trees (3 ‘Arbequina’, 4 ‘Arbosana’ and 4 ‘Koroneiki’, from a collection planted in field in November, 2013) were selected, all finishing an off year (avoiding low floral intensity due to inhibition by previous season fruit), but having already flowered in previous seasons. They were spaced 6×5 m and managed with regular pesticide sprayings and fertilization, including spraying of boron previously to flowering, and pruned in an open vase shape. Tree sizes are presented in Table 1. Trees evaluated here were less than 40 m far from each other, and in this range other varieties flowered only in 2020, but in low intensity. Another olive varieties collection was sited 500 m away.
Evaluations were performed in the seasons 2018/19, 2019/20 and 2020/21. Prior to buds leaving autumn/winter rest (May to June), in each season, 120 shoots of each variety, divided equally among the plants, were selected and marked. The shoots were terminal, formed in the same growing season, with an inclination of less than 45º in relation to the horizontal, measured from 5 to 35 cm in length (Table 1), and located in the middle height of the crown. For each shoot, the length and the number of buds were registered (Table 1). The shoots were observed weekly since the last week of June to identify the beginning of inflorescence development.
Meteorological observations from automatic weather station 1041 - Epagri (Chapecó, 27,09°S/ 52,64°W, 687 m of elevation)19 are presented in Figures 1, 2 and 3 in Supplementary material.
2.1 Flower and fruit quantity
Each shoot was observed for the number of inflorescences, flowers or fruits from beginning of inflorescence growth until harvest. Field data were used to calculate the following indexes: percentage of flowering shoots, inflorescence number per meter of shoot, percentage of reproductive budburst, number of flowers per inflorescence, and fruit set. Fruit set was calculated taking the fruit counts when the fruit drop curve stabilized (December 5, 2018; November 11, 2019; November 13, 2020), and dividing by the maximum number of flowers registered.
2.2 BBCH phenological stages
Assessment of phenology was made based on the BBCH phenological scale20, on a weekly basis at flowering and maturation phase and every two weeks at fruit growing, whenever possible. For each shoot, inflorescences were observed individually, and the predominant, the most advanced, and the most delayed stages were written down. The evaluation was completed in the harvest phase (stage 80 or more advanced).
2.3 Data analysis
Data on percentage of reproductive budburst, inflorescences per meter of shoot, fruit per inflorescence and fruit set were averaged in each tree. In each season, all parameters were submitted to analysis of variance (ANOVA, α=0.05) having cultivars as factor, in a completely random design, where the experimental unit was each individual tree (3 repetitions of ‘Arbequina’, and 4 of ‘Arbosana’ and ‘Koroneiki’). When significant, the ANOVA was complemented by a Tukey test.
3. Results
3.1 Flowering and fruiting indexes
The percentage of flowering shoots varied throughout years and cultivars (Table 2). In ‘Arbequina’ and ‘Arbosana’ it grew over the years, while in ‘Koroneiki’ it was more stable, but still reaching from 13.33 to 72.57%. In 2020/2021 94% of ‘Arbequina’ shoots flowered, while the lowest flowering shoots percentage was observed in ‘Arbosana’ in 2018/2019. Flowering shoots percentage in ‘Arbosana’ was significantly lower than in ‘Arbequina’ the last two seasons.
The percentage of reproductive budburst and inflorescence density, given as the number by meter of shoot, followed the same trend seen in the percentage of flowering shoots. The lowest values were found in ‘Arbosana’ (2018/2019) and the highest in ‘Arbequina’ (2020/2021) (Table 2). In 2018/2019 ‘Arbosana’ emitted just one inflorescence in all 120 shoots evaluated, so those data will be ignored in the rest of section 3.
Table 2: Percentage of flowering shoots and reproductive budburst, number of inflorescences per meter of shoot, number of flowers per inflorescence (FPI), and fruit set in 3 olive cultivars and growth seasons (Chapecó, Brazil)
I Differences inside the harvest season are not significant (ANOVA, α=0.05). II Means followed by the same letter inside each harvest season are not significantly different (Tukey, α=0.05). III Not determined - absence of fruit in the tagged twigs. IV It was not feasible to determine a time to calculate it because fruit drop occurred continuously until ripening.
3.2 Reproductive phenological stages
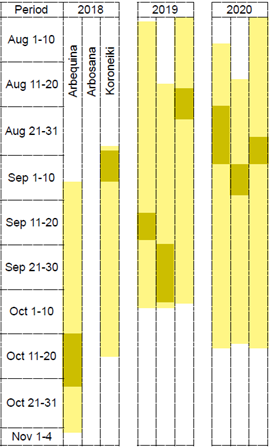
The phenological stages observed for each evaluation date and cultivar are presented in Figures 1 and 2. The earliest flower opening (BBCH 60) occurred between July 30 and August 8, 2020, in ‘Arbequina’ and ‘Koroneiki’, and between August 14 and 20, 2020, in ‘Arbosana’, which was the latest in all evaluated years (Figure 2). Opening flowers continued to be observed for a long period: in 2020, ‘Arbequina’ emitted new inflorescences from June 24 to September 25, a 93-day period. The shortest period was with ‘Koroneiki’ in 2019: 53 days. Therefore, the open-flower period (stages 60 to 68) lasted 41 to 68 days (excepting ‘Arbosana’ in 2018).
The stage 80 (deep green fruit getting light green or yellowish) was observed first in ‘Arbequina’. ‘Koroneiki’ had the last evaluated inflorescence to reach stage 80 in two seasons (Figure 1). The dates when all ‘Arbequina’ and ‘Koroneiki’ fruits reached 80 or a more advanced maturation stage were February 5, in 2019, and in 2020, January 9, 16 and 23, for ‘Arbequina’, ‘Arbosana’ and ‘Koroneiki’, respectively. In the 2020/2021 season, Arbosana's fruits were all in stage 80 still in December 23, 2020, while ‘Arbequina’ and ‘Koroneiki’ needed more time (January 8 and 15, 2021, respectively).
Figure 1: Occurrence of reproductive phenological stages of BBCH scale (left axis), and percentage of flowers or fruits persistent in the shoots (right axis), evaluated along 3 reproductive seasons in ‘Arbequina’ and ‘Koroneiki’, and 2 seasons in ‘Arbosana’ olive trees, in Chapecó, Brazil
4. Discussion
Flowering shoots varied in quantity throughout years and cultivars. As the percentage of flowering shoots increased, the percentage of reproductive budburst and inflorescences by meter of shoot increased too, i. e., the floral intensity incremented. The result is a higher number of flowers emitted in a canopy volume unit, leading to bigger fruit yield (Table 1). The variation among years in the same cultivar was higher than the variation among cultivars in the same year, which suggests that climate conditions and tree age may be the main factors influencing flowering intensity.
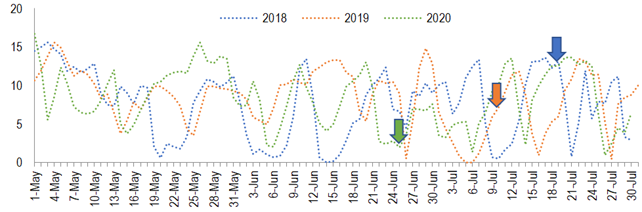
In Chapecó and other close, similar sites, the 3 cultivars were capable of flowering18 and fructify17 in the third season (less than 3 years it the field). Furthermore, in the Mediterranean temperate climate of Cyprus, tree yield increases until the fourth year. From then on the yield decreases or is maintained21. Those evidences suggest that the 5-year-old trees in Chapecó had already overcome juvenility in the start of the study. So, the inter-year variation observed in flower density is probably due to climatic conditions. This thesis is supported also by the higher counting of days with minimum temperature under 16 ºC (75, 63 and 94 in 2018, 2019, and 2020, respectively). In Greece, under Mediterranean temperate climate, a reduction in the number of flowers per ‘Koroneiki’ tree happens when chilling is reduced from 1,835 to 1,214 hours under 16 °C22. It has been demonstrated that when ‘Arbequina’ and ‘Koroneiki’ are exposed to high temperatures (23.9 to 26.6 °C) during the chilling period the intensity of flowering is lower23)(24. This may explain in part the low flowering intensity in Chapecó, since along May and June of 2018, 2019 and 2020 (chilling period), temperatures reached more than 23.9 °C in 25, 26 and 19 days, respectively. Using a chilling units model which discounts units as high temperatures occur, the chilling necessity of 234 units for ‘Arbequina’25 was not reached in any year. So, it can be hypothesized that insufficiency of chilling, even in the best year (2020), caused poor reproductive budburst, which is supported by the asynchrony in flower and fruit phenological stages26 (Figure 1).
‘Arbosana’ flowered scarcely, irrespective of the year, which suggests it needs more chilling than ‘Arbequina’ and ‘Koroneiki’. However, there is a lack of precise information on chilling requirements of olive cultivars in general. Irrespective of the cultivar, the number of inflorescences can be considered low to intermediate if compared to other regions: in the arid Egypt the cultivars Tofahi and Aggizi average in 2 years 66 inflorescences per meter of shoot27, which is more than all observations in Chapecó, except ‘Arbequina’ in 2020/2021; in Greece, ‘Koroneiki’ emits 60 to 80 inflorescences per meter of shoot along 3 years28.
The cultivars, in general, had a similar number of flowers per inflorescence, compatible with the observations in another humid, subtropical region - Barra do Ribeiro, Rio Grande do Sul29. In Spain, inflorescences of olive varieties including ‘Arbequina’ and ‘Koroneiki’ were evaluated in different environments with Mediterranean climate, showing a great variability in flower number. The environmental influence is bigger than the genetic effect30. ‘Koroneiki’ has inflorescences with more flowers than ‘Arbequina’ in some locations of Spain. However, ‘Arbequina’ showed more than 75% of perfect flowers irrespective of the environment31, agreeing with the superiority of ‘Arbequina’ over ‘Koroneiki’ in Barra do Ribeiro29.
All the varieties started the sprout from June to beginning of August, depending on the season. In Uruguay, a region with humid, temperate climate, across 10 years of observation in ‘Arbequina’, the earliest sprout happened in mid-August32. The implication of early sprouting is the risk of low, positive temperature and frost damage to inflorescences, since in Chapecó and adjacent areas frosts are expected until September33. In addition, in Uruguay, ‘Arbequina’ flowers in a window of 52 days, shorter than in Chapecó.
The flowering time of ‘Arbequina’ and ‘Koroneiki’ was also earlier than the observed in the subtropical climate of Bagé, Brazil, (31°19’53'' S, 212 m elevation)34, probably because in Chapecó temperatures tended to be higher during the winter, which started up inflorescence growth more precociously35. As an example, July average temperature in the 3 years varied from 15 to 17 ºC in Chapecó, and from 10 to 12 ºC in Bagé, despite different years.
While in Chapecó full flowering occurred around the 50th day of the second semester, in colder places of Europe it takes 13023) to 15026 days in the equivalent semester (the first).
Flowering duration varied from 50 to 68 days (excepting ‘Arbosana’ in 2018), agreeing with observations in a subtropical climate (Canary Islands), but longer than in a typical Mediterranean climate (Andalucía)26, and cooler places, such as Salto and Las Brujas (Uruguay), where ‘Arbequina’ flowering time lasts 6-18 and 7-28 days, respectively32. In Canary Islands, the trees show high amplitude of phenological stages at the same date26, similar to the observed in Chapecó (Figure 4 in Supplementary material).
Figure 2 illustrates how flowering time varied between years and cultivars. During the 3 years, the 3 cultivars flowering time overlapped in their majority, but the time when flower opening concentrated was not the same, except for ‘Arbequina’ and ‘Koroneiki’ in 2020.
Differences in full flowering date are common even in colder climates, as in Ubeda, Andalucía, where full flowering date varies even 26 days in ‘Arbequina’ between years26. In southern Spain, ‘Arbequina’, ‘Arbosana’ and ‘Koroneiki’ under several Mediterranean environments show small differences in full flowering date. Even though it is later in ‘Arbosana’ than in ‘Arbequina’, partial coincidence of full flowering period and overall flowering period is observed among them30. So, maybe those long and different flowering periods in Chapecó could be due to the warmer climate.
The poor synchrony among varieties can result in low pollen amounts suspended in the air the time most flowers are open, making cross-pollination less likely. This can be crucial for ‘Arbequina’, which is considered not self-compatible36)(37. In Rio Grande do Sul, bagged ‘Arbequina’ shoots set fruits, but less than no bagged ones29, which means it benefits from cross pollination.
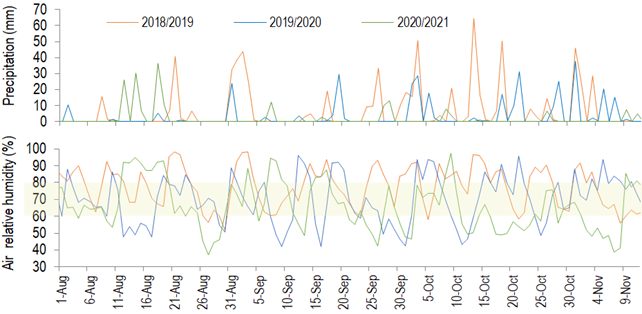
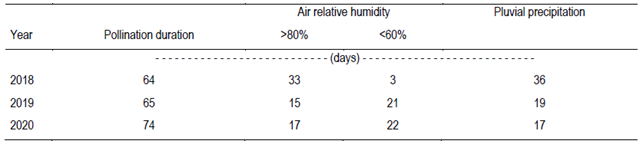
In the years when fruit set was higher, tree yield was lower, and the opposite relation was observed with flowering intensity and yield (Table 1). In years with intense olive flowering, only 1 to 4% of the flowers become fruit, due to the high competition for assimilates7. This index can reach 10%8. In the arid Chaco, Córdoba, Argentina, 3.6% of fruit set results in more than 80 kg tree-1 of fruit (70-year-old ‘Arbequina’ trees, 10×10 m spacing)38. In Barra do Ribeiro, fruit set is not determinant for fruiting29. Further, during the pollination period in Chapecó, days with average air relative humidity above 80% or lower than 60% were slightly predominant (Table 3 and Figure 5 in Supplementary material), which probably did not harm pollination in a way to avoid a good fruit set. Therefore, based on the present observations, it is not possible to state that the fruit set limits the yield in Chapecó.
Table 3: Number of days with maximum air temperature higher than 25 ºC, with average air relative humidity higher than 80% or lower than 60%, and with pluvial precipitation in Chapecó along the olive pollination period in 3 years
Olives can be picked when fruits turn deep green to yellowish green, which is equivalent to the BBCH stage 8020. In Chapecó, the latest cultivar to reach harvest time was ‘Koroneiki’, contrasting ‘Arbequina’, in accordance with results in Croatia39, a colder site. In Minas Gerais, in a higher altitude (1,276 m) compared to Chapecó, ‘Arbequina’ harvesting time starts on January 20 to 2540, later than in Chapecó. The precocity in fruit maturation can be explained by the early flowering, low fruit load (which accelerates the maturation41), and quicker accumulation of degree-days42 compared to colder regions.
Early harvest helps to avoid higher air humidity times in the end of summer and autumn33, which can ease losses by anthracnose caused by Colletotrichum sp.43. Nevertheless, a long flowering period leads to variability in fruit ripening stage in a same tree. Growers will have to wait to harvest an entire tree when almost all fruits reach a minimum maturity, when others could have reached advanced stages, which is a risk of high acidity and low polyphenol content in the oil, especially in trees with low fruit load41. In addition, higher temperatures and solar radiation during oil synthesis and accumulation could cause an undesirable balance in oleic versus linoleic acids, and lower oil accumulation44)(45.
5. Conclusions
When the olive cultivars Arbequina, Arbosana and Koroneiki are grown in the conditions of Chapecó, Santa Catarina, Brazil, inflorescence budburst occurs early, from end of June to end of July, flowering from early August to early November, and maturation starts from end of December until early February. In comparison to traditional olive growing regions, flowering intensity is low in some years as a direct effect of climatic conditions; nevertheless, fruit set is similar.
Although the evaluated cultivars produce fruits under Chapecó conditions, the lack or low intensity of flowering associated to sprouting in a time with risk of frosts will probably make growers to experience unstable olive harvests, sometimes null, which discourages their initiative in this region.