1. Introduction
Honey is a natural sweet substance produced by bees Apis mellifera from plant nectar, from secretions of live parts of plants, or from excretions of insects that feed on live parts of plants and which bees collect, process, and combine with specific substances of their own, and deposit, dehydrate, store and leave in the honeycomb to mature and age1.
The most common resource used by bees in honey production is flower nectar. However, there are other extra-floral sources of secretions of sap-sucking insects, mainly Aphididae2. The honey production of Apis mellifera in Uruguay is one of the main farm products, marketed, in more than 90%, to Europe and North America3. These honeys must be subject to a continuous routine analysis, which demonstrates their genuine origin, their quality in terms of physicochemical parameters and free of contaminating residues. Some studies have characterized Uruguayan honeys4-12. But the physicochemical approach in the northern region of the country is still incipient. Studies that refer to honeys from the northeast of Uruguay do not contemplate the production of the department of Rivera6. The development of livestock and forestry production in this agricultural-free region allows both local (non-transhumant) beekeepers and those who move from agricultural (transhumant) areas to produce eucalyptus honey. An area of natural forest known as Valle del Lunarejo is associated with this region, which belongs to the National System of Protected Areas (SNAP by its Spanish acronym). It is possible to find combinations of soil, climate and vegetation in this area that allow producing typical honeys at different times of the year. These honeys have shown to differ in their botanical origin, with the presence of myelates11-12.
Glyphosate contamination does not seem to be a threat in both, protected area and afforestation, due to the production system itself; however, it is necessary to take specific precautions in each harvest season. Characterizing the honeys of this region is fundamental to create new marketing opportunities with a differential product, with characteristics typical of the place and free of contaminants. This study aimed to characterize, through physicochemical parameters, botanical origin and contaminant residues (glyphosate), honey produced in forest systems of Eucalyptus grandis (monofloral) and honey produced in the protected area Valle del Lunarejo.
2. Material and Methods
2.1 Sampling
Samples of honey from the northeast of Uruguay from forest production of Eucalyptus grandis were analyzed under two beekeeping production systems, without transhumance (EWM) and with transhumance (EM) in the autumn 2019 season, and from the native forest of the Valle del Lunarejo protected area (PA), in 2019 spring season (Figure 1). Ten beehives were randomly selected at each production site, and two frames with wax sheets were introduced into each beehive. After these were worked and capped, pieces of honeycomb were cut and placed in sterile jars for further analysis. This sampling method ensures the freshness of the honey, the production period, and the geographical origin, in addition to controlling the techniques applied in the apiary (cures, feeding, etc.)11.
2.2 Botanical and physicochemical determination
2.2.1 Palynological analysis
Palynological analysis was performed according to the Louveaux method13. After centrifuging, the residue was observed under a 400 magnification microscope, and 600 pollen grains were identified and accounted for to determine the relative abundance of each determined rate14. The honeydew elements (HDE) were determined and quantified to relate them to the abundance of pollen grains.
2.2.2 Minerals
Minerals were determined by flame (Ca, Mg) or emission (Na, K) Atomic Absorption Spectroscopy with an EAA Perkin Elmer PinAAcle 500 (USA). A liquid sample was aspirated, aerosolized and burned in flame for the release of specific minerals15. One gram of honey was taken to acidic solution, with ultrapure HNO3 (1M) and HCl 6 M, in Erlen with steam trap device, in hot iron, for one hour, and then brought to volume with distilled H2O, less than 18 Mohms16. A standard curve was constructed for each analyte from Ca, Mg, K and Na stock solutions of 1000 mg/l Merck (Germany). The content of Iron (Fe), Zinc (Zn), Copper (Cu) and Manganese (Mn) in honey was also quantified, with an Atomic Absorption Spectrometer (Perkin Elmer, AAnalyst 300, USA), equipped with a deuterium lamp as background corrector, with flame (air-acetylene; 8.0 l/min and 1.4 l/min).
2.2.3 Moisture, conductivity, and pH
The moisture content was measured with a 3 M ATAGO-MASTER manual refractometer (58° to 90° Brix), using a drop of honey from each sample. Data were expressed as moisture percentage (%). Conductivity and pH were measured in a dilution of 20% (w/v) of deionized water. Electrical conductivity was measured with a conductivity meter OAKTON CON 10 (USA). Conductivity was expressed in μS/cm. The pH was measured with a pH meter JENWAY 3305 (UK), at 20 °C room temperature.
2.2.4 Color
The honey extracted from the honeycombs was centrifuged in a centrifuge (Thermo Scientific, Sorvall ST-16R, USA) for 10 min at 3000 rpm. Once centrifuged, a disposable bucket for spectrophotometry of 4 cm³ and 1 cm wide (PS, PlastiBrand™, Germany) was filled. The bucket was covered and placed horizontally on a white background on a countertop illuminated with fluorescent light tube (T8, 36 W, cold - Philips L24540). The color was measured in three areas of the bucket, in the CIELAB color space with a colorimeter (Konica Minolta CR-10, Japan), illuminant D65, observer angle 10º and illuminant angle 8º. The variables of color were measured: luminosity (L*), a* (+a* red, - a* green) and b* (+b* yellow, -b* blue), and tone (Hue, ºHab) and saturation (Chroma, C*ab) were calculated. A classic color determination was made in mm Pfund, so as to compare. It was determined using a HANNA digital colorimeter, with direct reading on the 4 cm³ buckets.
2.2.5 Sugars
The sugars: fructose, glucose, sucrose, mannose, galactose, isomaltulose (palatinose), maltose and trehalose were determined by high-performance liquid chromatography (HPLC). One gram of honey was taken from each sample, which was stirred with 20 mL of deionized H2O to homogenize. The solution was filtered before injection into the HPLC using PVDF filters of 13 mm diameter and 0.45 microns of pore size (Merck Millipore). An HPLC Prominence, LC-20A series, Shimadzu Corporation (Japan) was used, equipped with a differential refractive index detector (RID-20A), integrated solvent supply pump with quaternary gradient kit (LC-20AT), autosampler (SIL-20AC HT), column thermostatization oven (CTO-10AS VP) and degassing unit (DGU-20A 5R).
LabSolutions software was used for data processing. A Column Luna Omega SUGAR 100 Å, 3 μm, 250x4.6 mm (Phenomenex, USA) was used for separation, thermostatized at 40 ºC. The mobile phase consisted of Acetonitrile:H2O (80:20) at a flow of 1 mL min-1. The RID detector was thermostatized at 40 ºC. The total running time was 30 min. Quantification was carried out with external standards: D(-)-Fructose (99.9%, Certified Reference Material, Sigma-Aldrich, USA),: D(+) -Glucose anhydrous AR® ACS (97-102%, Macron Fine Chemicals, USA), D(+)-Sucrose (99.5%, Sigma-Aldrich, USA), Isomaltulose (94.5%, USP Reference Standard, USA), D(+)-Galactose (≥99%, Sigma-Aldrich, USA), D(+)-Mannose (≥99%, Synthetic, Sigma-Aldrich, USA), D-(+)-Maltose monohydrate (≥99%, Solanum tuberosum, Sigma-Aldrich, USA) and D-(+)-Trehalose dihydrate (≥99%, from Saccharomyces cerevisiae, Sigma-Aldrich, USA); generating respective calibration curves for each sugar.
2.2.6 Glyphosate
Glyphosate + AMPA content was determined from 50 g of a composite sample of honey. The reference technique was based on the Spanish standards17. Results are expressed in mg/kg honey (ppm). The detection limit is 0.05 mg/l and the quantification limit is 0.2 mg/l.
2.3 Statistical analysis
The data obtained were analyzed with a simple ANOVA and the mean differences, by the Tukey-Kramer test (p<0.05). Homogeneity of variances and normal distribution of the analyzed variables are assumed. Two multivariate analysis tools were used: cluster analysis (dendrogram) to evaluate the similarity between the samples obtained, and principal component analysis (PCA) to look for possible associations between physicochemical and nutritional variables and the honeys studied. The dendrogram was performed with the average Manhattan distance and Ward's grouping method using the variables Luminosity, pH, conductivity, sugars and minerals. The Infostat program was used18. The PCA was performed with the same variables. The ANOVA and the PCA were carried out with the RStudio program19.
3. Results
3.1 Honey samples
A total of 27 honey samples were obtained, 10 samples were from EWM, 8 from EM and 9 from PA. The number of samples from each production site is different since the frames were not 100% capped in EM and PA at the time of harvesting.
Table 1: Relationship between pollens and honeydew elements (HDE) of each honey sample
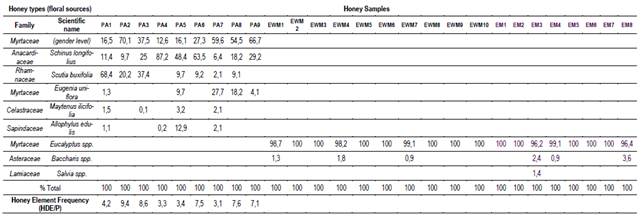
Data represents HDE/pollen ratio and percent of pollen in each honey sample.
3.2 Palynology
The result of the palynological analysis is presented in Table 1. The honeys obtained in EWM and EM presented a very high percentage of Eucalyptus sp. (dominant pollen > 95%), agreeing with the monofloral requirements. These samples were obtained between March and April, over 15 days, when most of the supers were completely capped (10 kg). Honey samples from the PA site produced in spring (September to December) have a greater diversity of botanical origins, highlighting the presence of Anacardiaceae and at least two species of Myrtaceae (Table 1). These honeys (PA) showed the presence of abundant honeydew elements and a ratio of HDE/pollen>3, characteristic of myelates honey13.
3.3 Minerals
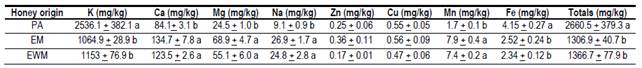
Results of the mineral content are observed in Table 2 and are within the range reported by other authors. Honey from the United States of America has an average value of 1700 mg/kg of minerals in honey20; in Europe, it presents values of 1000 to 2000 mg/kg in floral honeys and more than 10000 mg/kg in myelates21.
3.4 Moisture, conductivity, and pH
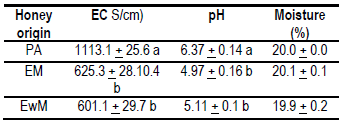
The data obtained are presented in Table 3. No significant differences were found for moisture between honeys. The values were within the range cited in the literature and accepted for honey1. The electrical conductivity analysis showed no significant differences between the EWM (601 ± 29.7 µS/cm) and EM (625.3 ± 28.1 µS/cm) honey; however, these results were significantly different to the PA value (1113.1 ± 25.6 µS/cm). No significant differences were found in the pH values for EWM (5.11 ± 0.1) and EM (4.97 ± 0.16) honey, respectively; however, these were significantly different from PA honey (6.37 ± 0.14).
3.5 Color
The results obtained for color, measured through the mm Pfund scale and by CIE L*a*b methodology (Table 4), show that there is a similarity between the eucalyptus honeys of both production systems, EWM: 75 mm and EM: 81 mm, being in the shade of Light Amber. The PA honeys had a darker coloration, 92 mm, which is classified as Amber.
3.6 Sugars
The data obtained are presented in Table 5. There were no significant differences between EWM and EM honeys, however, they differ significantly from PA honey in the contents of disaccharides: isomaltulose, maltose and trehalose, and in the mannose monosaccharide.
3.7 Glyphosate
No presence of glyphosate was detected in the honey samples analyzed in the 3 sites, EWM and EM (forest) and PA (native forests).
3.8 Descriptive statistics
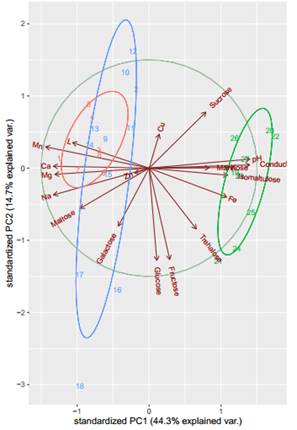
Two groups of honey can be observed through cluster analysis (dendrogram). On the one hand, eucalyptus honeys that, regardless of the site and the form of production, are associated with similar characteristics. On the other hand, honeydew honeys, in addition to being a single group, were closer to each other (Figure 2). Regarding the PCA multivariate analysis, the contents of Mn, Ca, Mg, Na, Maltose and Galactose were found to be the variables that best explain the groupings of the EWM and EM honeys, while for PA honeys the Fe, pH, conductivity, Isomatulose and Mannose had a greater incidence (Figure 3).
4. Discussion
4.1 Palynology
The abundant floral supply and the proximity of these floral sources to the EWM and EM apiaries explain the absolute predominance of eucalyptus pollen. The forestry system does not allow the implantation of other plant species within the forest, thus, some other species are found on the periphery of the afforestation (Baccharis sp.). PA honeydew honeys take more time to produce and are botanically more diverse. Beyond the extended production time, which allows the flowering of numerous species (Table 1), this demonstrates the richness and diversity of the protected area. These honeydew honeys with diverse floral pollen contents could be valued for their high mineral content and antioxidant properties2.
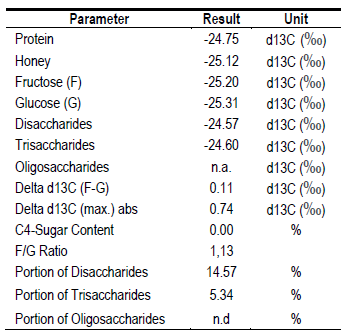
To ensure its origin, a complementary analysis of carbon isotopes was performed22)(23. It was determined that its origin is from C3 plants (Table 6), since they do not exceed the limit value (Δδ13C ± 2.1 ‰) and the F/G ratio (Δδ13C ± 1.0 ‰)22. Discarding a possible adulteration by use of syrups. Although its specific origin is not known, it is possible that, due to its high content of K and Maltose, it comes from some species of Fabaceae. The Bracatinga (Mimosa scabrella, Fabaceae) of the southern region of Brazil generates a myelate with these characteristics24. Beak-sucking insects that feed on C3 species are likely present in this region, generating myelates. Recently, it was discovered in Uruguay that the Flatidae Epormenis cestri, when fed on Sebastiania schottiana, excretes a substance that bees collect, making a honeydew honey with high pH and conductivity25.
4.2 Minerals
The EWM and EM honeys presented similar and even higher mineral content than other eucalyptus honeys26)(27, despite the fact that this study determined only 8 minerals of the 54 possible minerals detected in honey27. Significant differences were found between eucalyptus honey (EWM and EM) and PA honey for minerals K, Ca, Mg, Na, Mn and Fe. The lower Na content (9.1 mg/kg honey) in PA honey compared to EWM and EM is highlighted.
Table 4: Means and standard deviations of the color variables of the 3 honey groups
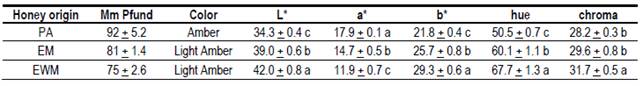
Different letters in each column mean significant differences p> 0.05 (Tukey-Kramer).
Table 5: Means and standard deviations of the analyzed sugars in the 3 honey groups
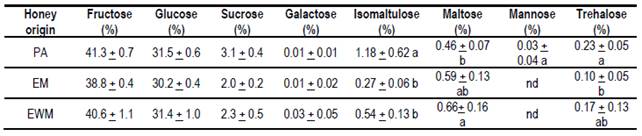
Different letters in each column mean significant differences p> 0.05 (Tukey-Kramer). nd = not detected
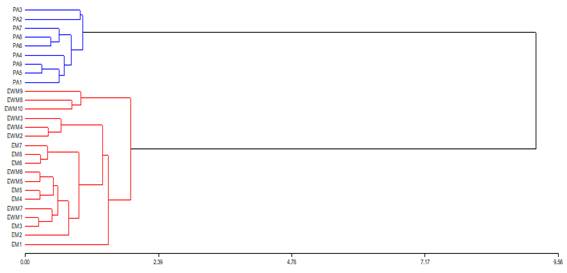
Figure 2: Dendrogram. In blue, honeys from protected area; in red, both groups of honeys from Eucalyptus grandis
This value is lower than others found in this same region11. The higher content of Fe also stands out (4.15 mg/kg honey) in PA honey, which coincides with another study for this same region12. The Mn content is very low in honey in general21)(27. PA honey had lower content (1.7 mg/kg), but matches previous results reported for the region12. The content of these minerals is relevant in the human diet since they are members of enzymes such as catalase (Fe) and superoxide dismutase (Mn), which act by preventing oxidative stress at cellular level27. These differences found in the mineral profile are attributed to the different groups of soils and vegetation existing at each site28.
4.3 Moisture, conductivity and pH
Regarding the moisture of honey, although the rules establish a maximum, exceptions are accepted. Bibliography cites honeys from desert climates with less than 14% moisture29 and honeys from humid tropical climates with values over 27%30. Working with honeys extracted from 100% capped honeycombs ensures the minimum moisture bees are able to achieve in the climatic conditions of the region31.
The European limit for electrical conductivity applied to floral honeys is 800 μS/cm, this reaffirms the extra-floral origin of PA honeys, values associated with high mineral contents28. Other floral honeys (with the presence of myelates) of the region (PA) presented high conductivity values (920 μS/cm) associated with high values of K (3150 mg/kg honey)11. The conductivity values of EWM and EM honeys (600 μS/cm) were higher than other values reported for eucalyptus honeys with fewer minerals (480 μS/cm)27. This greater amount of minerals could mean a differential feature of the eucalyptus honeys of Uruguay. Eucalyptus honeys from other regions of Uruguay have been reported to contain higher conductivity values than those found in this study10. This could be explained by differences in soils (fertility, depth and mineral content) or by eucalyptus varieties.
The pH values found in PA honeys (6.37) have not been previously reported in Uruguay7 and differ from previous studies carried out in this same region and the same harvest season (spring). The mentioned study found honeys with myelates, but of floral origin of Schinus, Lithraea brasiliensis, Myrcianthes sp. and Gleditsia sp., with an average pH of 4.211. This difference could indicate that different honeys can be produced in the same region and season of the year due to a year effect, and it can give them greater value for their originality. These characteristics could be typical of this place, associated with the botanical and geographical origin. At medicinal level, honeys with high pH values (up to 6.97)29 acquire value due to their use in treatments of gastric ulcers32, so, evaluation of these myelates through these physicochemical and nutritional characteristics should continue.
4.4 Color
All honeys presented shades of color that are the most accepted by the Uruguayan consumers8. Pfund values, as well as the mineral content in EWM and EM honeys, are higher than those reported for eucalyptus honeys27)(33. In Uruguay, values of 62 to 75 mm Pfund were found in honeys of Eucalyptus sp. with L* values from 32.6 to 34.38. These L* values are lower than those found in this study (Table 5). The way the samples are obtained, the storage period and temperature, and the lighting conditions when measuring could explain these differences. Eucalyptus honeys in the world present different color ranges in mm Pfund (53 mm to 96 mm and 41 mm to 77 mm), but the differences between species must be considered33. L* values measured through the CIE L*a*b methodology (Table 5) were within the range reported for other Uruguayan honeys11. They were significantly different, being the honeys of EWM more luminous (42.0) than EM (39.0) and PA (34.3). This difference in L* occurs even though the EWM and EM honeys did not present differences in the total mineral content and belong to the same scale according to Pfund. This supports the increased ability to separate honeys through color by the CIE L*a*b* method.
4.5 Sugars
While the honey samples obtained in EWM and EM were obtained from the production of Eucalyptus grandis, the values of fructose, glucose, the sum of both (F+G), and sucrose are within the ranges reported for eucalyptus honeys Eucalyptus globulus34 and Eucalyptus camaldulensis35. These values are within the accepted ranges proposed in standards for eucalyptus floral honeys1. Apart from some differences in mineral content and conductivity, these honeys can be determined as eucalyptus by their sugar content.
4.6 Glyphosate
The absence of glyphosate in EWM and PA honeys could be due to local beekeepers not producing outside the region. These results coincide with an older study conducted at the PA site, where the presence of glyphosate was also not detected11. There is a low risk of contamination due to the productive systems (livestock and afforestation) of the region11. The absence of glyphosate in EM honeys does not imply that there is no risk of contamination if the beekeepers were previously in agricultural areas. It is necessary to insist on the good management practices that must be carried out by transhumant beekeepers in agricultural areas and when they arrive from them.
5. Conclusions
Through this study, typical eucalyptus honeys from the northeastern region of Uruguay and honeys from the native forest of the protected area have been characterized physicochemically. The productive potential of eucalyptus in that region and the quality of the honey obtained are highlighted. The volumes produced in a short time (10 kilos per hive in 15 days) justify transhumance into this crop, obtaining contaminant-free honeys. In the native forest of the protected area, another type of honey is produced, of extra-floral origin, with particular characteristics that could enhance it. Although the production period is longer and the volumes produced are smaller, the originality of that honey makes it different and can be an argument for the maintenance and expansion of protected areas. Subsequent research should study the identification of insects, and from which plants these honeydew honeys are generated and what antioxidant properties they could have. Within the protected area it is possible to obtain honeys with different characteristics compared to other regions, which should be studied for a longer time and in greater depth.











 Curriculum ScienTI
Curriculum ScienTI