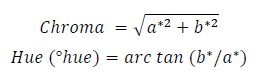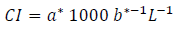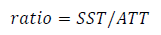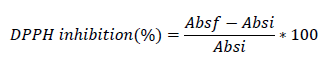1. Introduction
The butia is the fruit of palms of the genus Butia Becc. of which there are at least 20 reported species, among them Butia odorata (Barb. Rodr.) Noblick1. These palms are part of a very particular ecosystem distributed in the area near the Río de la Plata in southeastern Uruguay, Argentina and up to northwestern Brazil2)(3)(4. The fruit has cultural, scenic and economic value, and is traditionally used for consumption and other uses by the inhabitants of the protected area of Los Palmares de Rocha, Uruguay3)(5)(6)(7)(8)(9. These palm groves make up biosystems declared national heritage and are part of the Bañados del Este World Biosphere Reserve5)(6)(7.
The fruits are eaten whole in natura and used in different preparations, such as jellies, sweets, liqueurs, ice creams, sweet and sour sauces, and chocolates8)(9)(10)(11)(12. Most of these products include only the pulp8)(10)(11, leaving the peel as a possible by-product, whose use is not yet defined. On the other hand, the palms from which the fruits are obtained are more than 100 years old and are located in livestock and agricultural production systems (rice) in which their natural replacement is very difficult3)(4)(5)(7)(13)(14)(15. One of the ways to preserve the palms and the biodiversity of these ecosystems is to find rational exploitation systems that include procedures for collecting and using the fruits4)(16. In the palm grove, some plants produce fruits with different epicarp colors from yellow to bright red4)(5.
Color is one of the first attributes that define fruit quality for the consumer. Pigments such as carotenoids, chlorophyll and phenolic compounds give color to fruits, providing nutrients and bioactive compounds with potential beneficial effects on human health17)(18)(19)(20)(21)(22)(23. On the other hand, most of the studies in butia fruits do not differentiate the composition of the peel and the pulp. Consequently, it is of interest to know and consider the chemical, nutritional and bioactive properties that these native fruits have, which can value their use and differentiate by-products such as the peel.
The study aimed to characterize chemically and physically the peel and pulp of fruits of Butia odorata (Barb. Rodr.) Noblick with three epicarp colors (red, orange and yellow), by color, total titratable acidity, pH, total soluble solids, dry matter, ash, crude protein, dietary fiber, carotenoids and xanthophylls, vitamin C, total polyphenols, and total antioxidant capacity.
2. Material and methods
2.1 Plant material
The fruits were obtained in "Vuelta del Palmar" (Castillos, Rocha) in southeastern Uruguay, belonging to the Bañados del Este World Biosphere Reserve, 32° and 35° S latitude and 53° and 55° W longitude4)(5)(14. Palms with yellow, orange and red epicarp butia fruits were selected (Figure 1).
The three types of fruit colors were harvested in a mature state, washed and disinfected in an immersion bath in water with NaOCl (100 ppm) stirred for 5 min. Three kg of fruits of each color without visible damage, rotting and/or alterations, were selected and rinsed. Within each color, three replications were randomly obtained (≈1 kg/replication). In each repetition, 20 fruits were weighed and peeled manually with a scalpel, separating the peel (epicarp) from the pulp (mesocarp). The peel and pulp were weighed to calculate their relative proportion to the total weight of the fruit. The color of the peel and the pulp was determined in 20 other fruits, and the pulp juice was extracted using a domestic juicer (Philips, Hr1854) to measure the content of total soluble solids, pH and titratable acidity. The remaining fruits of each repetition were preserved whole in a freezer (-20ºC) to reduce the alteration of the chemical compounds of the peel and pulp that were subsequently analyzed.
2.2 Color
Peel and pulp color was measured instrumentally by the CIELAB system using a colorimeter (Minolta CR-10, Japan) at two opposite points on the equatorial zone of 20 fruits. The variables L* (Lightness, 0 = black to 100 = white), a* (-a = green, a = red) and b* (-b =blue, b = yellow) were determined, and the color saturation (Chroma) and hue (°hue) were calculated with the following equations 24:
In addition, a color index (CI) was calculated with the formula 25:
2.3 Soluble solids, pH, titratable acidity
The content of total soluble solids (TSS) was measured in the pulp juice with a digital refractometer (ATAGO Poquet PAL-10, Japan) expressing the data in °Brix26. The pH27 and the total titratable acidity (TTA) were measured in the same pulp juice by potentiometry in a 10 mL juice sample, with NaOH solution (0.1 N) and endpoint in pH 8.128. The results were expressed as a percentage of citric acid. In addition, the ratio between TSS and TTA was calculated as a measure of maturity index using the formula 29.
2.4 Dry matter, ash, protein and dietary fiber
The dry matter and ash content was determined from a 10 g fresh sample and dried in an oven (Blue.M, USA) with zenith ventilation at 105 ºC until constant weight. To determine the ash content, 15 g of fresh sample was incinerated in a muffle (Barnstead/Thermolyne, 4800 Thermo Scientifc, USA) at 550 ºC for 24 h. Data were expressed as a percentage of dry matter and ash. The content of dietary fiber and crude protein was quantified in the peel and pulp with the enzymatic-gravimetric method30 and as total N * 6.25 by the Kjeldhal method31, respectively. Data were expressed as a percentage of dietary fiber and protein in fresh base.
2.5 Carotenoids
The extraction and quantification of carotenoids were carried out according to Zaccari and others32 from 1 g of pulp and 0.5 g of peel in a tetrahydrofuran and methanol extraction solution (1:1 v/v; Mallinckrodt Baker, USA). The amount of α and β-carotene and xanthophylls (lutein, zeaxanthin and β-cryptoxanthin) was quantified by high-performance liquid chromatography (HPLC) (Spectral Series P100, Thermo Separations Products, USA), with a column C30 YMC TMCarotenoid S-5; 4.6 μm x 250 mm (Waters, USA), and a UV/Visible detector (UV-2000 SpectralSystem®, USA) set at a wavelength of 450 nm. The standards for the calibration curves were trans β-carotene type I (95%), a mixture of carrot carotene isomers (β:α 2:1, ≥ 95%); and xanthophylls of Tagetes spp. (α-carotene-3,3′-diol, β, ε-carotene-3, 3′-diol, β, ε-carotene, lutein, ˃75%), all Sigma Aldrich. The xanthophylls zeaxanthin (RT 5.02 min) and β-cryptoxanthin (RT 7.40 min) were identified by their retention time (RT) with regard to trans-β-carotene, according to Rodriguez-Amaya22) and Maurer and others33. Results were expressed in milligrams (mg) of carotenes and micrograms (µg) of xantophylls every 100 g of fresh weight.
2.6 Vitamin C
Vitamin C content was quantified according to Schaffert and Kingsley34 in UV-Visible spectrophotometer (Genesys10 VIS; Thermoelectro Corporation) at 521 nm wavelength, with some alterations. Briefly, the extraction was performed with oxalic acid (0.1% v:v) in 1 g of peel and 2 g of pulp quantifying the reaction of 2-4 dinitrophenylhydrazine (20 g DNPH L-1) acidified with H2SO4 (85%). The calibration curve was prepared with L-ascorbic acid (99.9%, Baker). The results were expressed in mg of ascorbic acid (AA) in 100 g of fresh weight of peel or pulp.
2.7 Total polyphenols
The total polyphenols were determined in the peel and pulp in a methanolic extraction using the Folin-Ciocalteau microscale protocol35 and at a wavelength of 765 nm in UV-Visible spectrophotometer (Genesys10 VIS; Thermoelectro Corporation). Data were expressed in milligrams of gallic acid equivalent in 100 g of fresh sample (mg GAE 100 g-1).
2.8 Total antioxidant capacity
The total antioxidant capacity was determined in the same extracts and with the spectrophotometer used to quantify the total polyphenol content. The total antioxidant capacity was determined as inhibition of the 1,1 diphenyl-2-picrylhydrazil radical (DPPH, Sigma Aldrich), by measuring the absorbance at wavelength 517 nm, based on the method described by Brand-Williams and others36. The determinations were performed in triplicate and expressed as a percentage of inhibition calculated with the following equation:
where: Absf is the absorbance of DPPH radical and Absi is the final absorbance of the sample.
2.9 Statistical analysis
The data obtained from the evaluated variables were analyzed using a one-way ANAVA with 95% confidence. The means were separated by Tukey's test (p ≤0.05) when applicable. Peel and pulp content was compared for the same color of fruit using Student's test (p≤0.05). In addition, the Pearson linear correlation (r, p≤0.05) was analyzed for peel and pulp, between the hue, the color index, the content of total polyphenols and the total antioxidant capacity, with the content of carotenoids and vitamin C.
3. Results and discussion
The fruits studied had an average weight of 9.3 g fruit-1, where the peel (epicarp) contributed 12.4% of the total weight, the pulp (mesocarp) 61.3%, and the core (endocarp and seeds) 26.3%. No statistical differences were found in the weight of the fruit, the peel, the pulp and the core of the three studied types of butia (data not shown). These results were similar to those reported in Butia capitata fruits for the epicarp and mesocarp (72.5%) and the endocarp and seeds (27.8%)4)(11)(15)(37)(38. Other authors11 determined that the epicarp constituted 64.5%, the mesocarp 10.8% and the endocarp with seeds 24.7% of the total weight of yellow and orange fruits. Fruit weight from 6.52 to 16.59 g was obtained in 11 butia genotypes with potential for genetic improvement, with 14.3 to 24.2% endocarp15. The fruit size is highly correlated with the weight of the pulp (r=0.93 to 0.98), which is useful to define the use of the fruits and to estimate the efficiency of industrialization processes4)(15.
3.1 Color
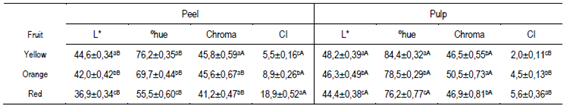
Table 1 shows the results obtained in the peel and pulp color. The peel of the butia fruits presented shades of orange-yellow color (55 to 76ºhue). The peel of the red fruits presented a dark orange value of instrumental color tonality and was less saturated than the orange and yellow fruits. On the other hand, the color tone of the pulp of the three types of fruits was less orange (76 to 84 ºhue) than the peel (Table 1). The color tones of the peel and/or pulp presented in Table 1 were similar to those reported by various authors in fruits of B. odorata, B. capitata and B. yatai harvested in the Pampa biome39)(40)(41)(42. These authors obtained peel and/or pulp colors with reddish-orange (46.49 - 68.4 º hue) and yellow (75.7 - 88.0 º hue) tones but with higher average lightness (L * 68.3 - 73.4)39)(40)(41)(42. Color variation in butia fruits, apart from being determined by the species (Butia Becc.), could be affected by the local edaphoclimatic conditions in which the palms develop42. In this regard, Ferrão and others42 observed that the color tone of the butia pulp in B. odorata was different according to the harvest region in Brazil, with light yellow pulp in the Santa Rosa region and dark orange in Santa María42. The color index (CI) allowed categorizing the fruits by the peel color in yellow with CI values ≈ 5; orange with CI ≈ 7 and red with CI values ≥ 14 (Table 1).
Table 1: Lightness (L), hue (ºhue) and saturation (Chroma) and color index (CI) of the peel and pulp of butia fruits
Mean ± SE (n = 40). In the same column, different lowercase letters indicate statistical differences between different colored fruits (Tukey p ≤ 0.05). For each color variable, different capital letters in the row indicate statistical differences between the peel and the pulp (Student's test, p ≤ 0.05).
Table 2: Content of total soluble solids (TSS), pH, total titratable acidity (TTA) and ratio in pulp juice of butia fruits
Mean ± SE (n = 3). Different lowercase letters in the same column indicate statistical differences (Tukey p ≤ 0.05).
Jiménez and others25 used this index (CI) as an objective measure to define citrus ripeness stages with peel color. The CI values were statistically different in the peel and the pulp, observing a greater range in the peel (5.5 to 18.9) than in the pulp (2.0 to 5.6). On the other hand, the pulp in the yellow fruits presented the lowest CI (2.0), with a higher and brighter yellow hue than their peel (5.5) (Table 3). The color tone and CI results could indicate a higher content of carotenoid and/or flavonoid pigments in the peel than in the pulp22)(43, in agreement with the data presented below (Figure 2, Table 3).
3.2 Soluble solids, pH, titratable acidity and Color index
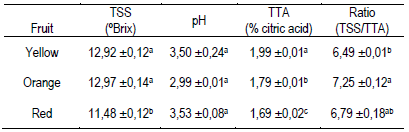
The TSS content and the pH of the pulp juice were similar between the three types of fruits (Table 2). They were also similar to the values reported by other authors, with TSS content of 9.33 to 15.5 ºBrix and pH of 2.93 to 3.9511)(15)(37)(39)(42)(44)(45. The titratable acidity expressed as a percentage of citric acid was lower in the pulp of red fruits, followed by orange and yellow fruits (Table 2). These results determined the ratio values calculated as TSS/TTA (Table 2), and together with the polyphenols in ripe fruit, it could be an organoleptic indicator associated with the particular bittersweet flavor of the fruit14)(15)(29)(46. The TSS in the juice of the vast majority of fruits are made up of sugars (80 to 90%), followed by organic acids and in a very low proportion: phenols, amino acids, proteins, fructans, minerals and water-soluble vitamins29. The results obtained by the ratio (TSS/TTA, Table 2) would indicate that the orange fruits appear sweeter to the consumers compared to the yellow fruits, although the TSS content does not differ from the other fruits. Organic acids such as vitamin C (L-ascorbic acid and L-dehydroascorbic acid) and vitamin B (folic acid and folates) are involved in the acidity of pulp juice and are of nutritional interest due to their action as anticoagulant and anti-inflammatory with impact on the cardiovascular and immune system47.
3.3 Dry matter, ash, protein and dietary fiber
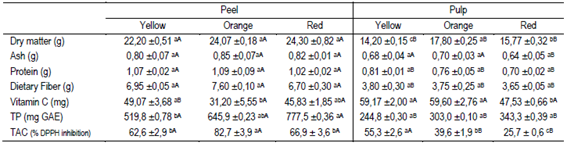
The dry matter content of the peel was similar between the three types of fruits. However, the pulp of orange fruits had the highest dry matter content (17.8%), followed by the pulp of red (15.7%) and yellow (14.2%) fruits (Table 3). The ash content was similar between the different butia colors, both in the peel and pulp. The peel and pulp of the butia types presented low protein content and high dietary fiber content (Table 3). In the peel, 47% more dietary fiber (≈7.08%) was determined than in the pulp (≈3.73%), similar to the results of a mixture of orange and yellow butia pulp (4.6% fiber)11. The peel contains more dry matter than the pulp, and probably, the structure of the epicarp cell walls has a higher proportion of hemicellulose, cellulose and lignin than the pulp tissues48. Fiber is considered to be very important in the diet, maintaining the body weight, acting in the prevention of some coronary diseases, reducing cholesterol, obesity, diabetes, lowering the postprandial concentration of glucose and the risk of colon cancer48)(49. The intake of 100 g of butia fruits in natura without peeling would provide 10 to 14% of the daily dietary fiber requirements of an adult50.
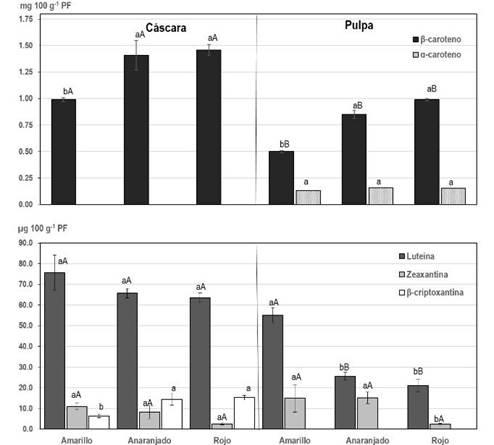
3.4 Carotenoids
The quantified carotenoid content per 100 g of peel and pulp is presented in Figure 2. The peel accumulated a higher content of total carotenoids (≈1.38 mg) than the pulp (≈0.97 mg). These results were consistent with a lower hue and a higher CI in the peel (Table 1, Figure 2), linked to the quantified carotenoids and their proportion in each matrix. In the peel, the content of β-carotene was 39% higher (≈1.28 mg) than that determined in the pulp (≈0.78 mg) (Figure 2). The peel of the yellow fruits presented the lowest content of β-carotene, differing statistically from the peel of orange and red butia. A similar result was obtained for the content of β-carotene in the pulp (Figure 2). In the peel, α-carotene was not detected in any of the three types of butia, while the content was similar in the pulps (≈0.15 mg). The lutein content was also higher in the peel (≈67.9 µg) than in the pulp (≈33.9 µg). The pulp of yellow fruits had twice the lutein than the pulp of fruits of other colors of butia. Other xanthophylls identified were zeaxanthin and β-cryptoxanthin. The zeaxanthin content in the peel and the pulp was similar between the three types of fruits, and β-cryptoxanthin was not detected in the pulp of any of the types of fruits analyzed (Figure 2). In the peel, the presence of a higher content of β-carotene and β-cryptoxanthin would contribute to pigment this part of the fruit with a more orange color22.
In pulp, similar results of β-carotenes equivalent 100 g-1 were obtained in yellow fruits of B. capitata from the regions of Brazil, Minas Gerais (0.88 and 1.6 mg)51 and B. pindo 1.83 mg52. Between 0.013 and 1.95 mg of β-carotene were obtained in B. capitata, B. eriosphata from Capão do Leão and Pelotas (Brazil)53, also quantifying β-cryptoxanthin (18.3 at 26.3 µg) and lutein + zeaxanthin (7 to 119 µg) in each species, respectively. The color of the fruits and the level of association with the type of chemical compounds they contain could constitute an indicator of nutrients and/or bioactive compounds of interest. In this regard, the results obtained from the three types of butia studied, show that the color tone (ºhue) and the CI of the peel and pulp had a high correlation with total carotenoids and polyphenols (Table 4). The correlation of tonality was negative with the content of β-carotene (r = -0.9088) and with the TP (r = -0.7202). A higher hue (90 °hue) is associated with the yellow color24, indicating less orange and/or reddish-orange pigments (β-carotene, β-cryptoxanthin and flavonoids), and/or a higher content of yellow pigments (lutein and flavonoids)22)(54. At the same time, the lutein in the pulp which colors yellow vegetables22)(54, had a high correlation with the color of the pulp (r = 0.9367). A lower correlation between the hue and the CI with the previously mentioned carotenoids (Table 4), and also a high correlation with the content of β-cryptoxanthin (r = -0.9068) and zeaxanthin (0.7886), were found in the peel (Table 4). On the other hand, in the biosynthesis of carotenoids in fruit and vegetables, α-carotene is a precursor of lutein, β-carotene is a precursor of zeaxanthin, and this, in turn, is a precursor of β-cryptoxanthin22)(54. The carotenoid amount and profile obtained in the peel and pulp of butia could be evidence of different metabolism rates of these compounds depending on the fruit compartment (peel or pulp). Furthermore, pigments that confer color to fruits and vegetables, such as chlorophylls, carotenoids and flavonoids, are involved in reducing oxidative stress in vegetables due to their antioxidant properties, and in some cases, such as α and β-carotene and β-cryptoxanthin, they are precursors of vitamin A22)(55. Xanthophylls such as lutein, although less present than carotenes, are of special importance for the protection of sight, bones, the nervous and cardiovascular systems, and have antioxidant, anti-arthritis, anti-diabetic, anti-inflammatory activities, among other functions56. The intake of 100 g of butia fruits in natura with peel would provide 8% to 16% of β-carotenes and 0.5 to 1% of lutein required daily by a child40)(57)(58.
3.5 Vitamin C
The content of vitamin C quantified in the pulp was 3.6% to 47.7% higher than in the peel. However, only the vitamin C was statistically different between the peel and the pulp of the yellow fruits. The peel of the orange fruits and the pulp of the red fruits had the lowest vitamin C content (Table 3). The amount of vitamin C in 100 g of whole fruits was 34.8 mg, 40.4 mg and 42.4 mg for the red, orange and yellow butia fruits, respectively. A wide range of vitamin C content is reported for butia fruits (17.61 to 70.44 mg AA 100 g-1 fresh), depending on the species, variety and environmental conditions in which the crop is developed, and also on the quantification method used40)(53)(59)(60. Vitamin C is essential in the human diet and is a powerful antioxidant that must be obtained from fruits and vegetables. L-ascorbic acid (AA) is the most active and abundant form of vitamin C, followed by dehydroascorbic acid38. The evaluated fruits (Table 3) had 1.5 to 2.4 times less vitamin C content (≈39.2 mg) compared to some fruits considered rich in this vitamin, such as oranges, 83.2; lemons, 74.3; apples, 60.0; strawberries, 65, and kiwis, 92 mg every 100 g-1 fresh60)(61. Intake of 100 g of butia in natura would provide 39% to 57% of the daily vitamin C requirements of an adult49.
3.6 Total polyphenols
The content of total polyphenols (TP) in 100 g of fresh weight of peel and pulp is presented in Table 3. The fruit peels had a higher content of TP (≈647.7 mg GAE) than the pulp (≈297.0 mg GAE) in the three colors of fruits evaluated. The TP content in the pulp was similar between the three types of fruits.
However, 20% and 33% were quantified in orange and red fruit peel, respectively, more TP than in yellow fruit peel. Similar TP contents were found in pulp of B. capitata with 260.4 mg GAE53; 493.6 mg GAE60; 280.5 to 380.5 mg GAE12, and in B. eriosphata 278.4 mg GAE44. Gallic acid has been reported as the main polyphenol in butia pulp followed by hydroxybenzoic acid12. However, some authors have found other flavonoids and phenolic compounds (epicatechin and chlorogenic acid) as predominant in exhaustive studies on Butia Becc. genotypes, where the species and the number of polyphenols in the fruits depend on the species and the geographical location23. The pulp of other fruits such as banana, oranges and peaches, with 90.4; 33.7 and 84.6 mg GAE, respectively, contains less TP than butia fruits43. Polyphenols are strong antioxidants, reducing oxidative stress during fruit growth, in the postharvest and in the subsequent handling62)(63. In turn, phenolic compounds color the peel and pulp of fruits, in shades of orange, red, blue, violet, and purple, and are responsible for other sensory characteristics such as astringency and bitter taste63. From the nutritional point of view, the study of phenolic compounds has gained relevance due to their effect on human health both for the antioxidant (beneficial) properties23)(64 and for being antinutrients, reducing the digestibility of proteins and the absorption of Fe and Cu, also highlighting that the excesses of phenolic compounds can trigger some types of cancer42)(62)(63.
Table 3: Content of dry matter, crude protein, dietary fiber, ash, vitamin C, total polyphenols (TP) and total antioxidant capacity (TAC) per 100 g in fresh weight of pulp or peel of three colors of butia fruits
Mean ± SE (n = 3) In the peel or pulp, different lowercase letters indicate statistical differences between different colored fruits (Tukey p ≤ 0.05). For the same fruit color, different capital letters in the row indicate statistical differences between peel and pulp (Student's test, p ≤ 0.05).
Table 4: Pearson's linear correlation coefficient (r) between hue (°hue), color index, total polyphenol content and total antioxidant capacity with the content of α and β-carotene, lutein, β-cryptoxanthin and zeaxanthin and vitamin C from the pulp or peel of butia fruits of three colors
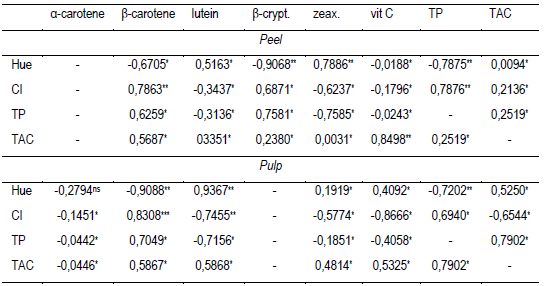
β-crypt.: β-cryptoxanthin: zeax.: zeaxanthin; vit C: vitamin C; TP: Total polyphenols; CI: Color Index; TAC: Antioxidant capacity. ns: not significant (p ≥ 0.05); * p ≤ 0.05; ** p ≤ 0.01; *** p ≤ 0.001.
3.7 Total antioxidant capacity
The red and orange fruits had 1.5 to 2.5 times more total antioxidant capacity (TAC) in the peel than in the pulp, while it was similar in the yellow fruits (Table 3). The peel of orange butias presented higher TAC than the peel of yellow and red fruits (Table 3). The pulp TAC was different between the three colors of fruits. The TAC was 1.4 and 2 times higher in the yellow pulp than in the orange and red pulp, respectively. The results obtained by comparing the TAC in the peel and in the pulp of orange or red fruits can be partly explained by the content of polyphenols, β-carotene and lutein (Table 3, Figure 2). Vitamin C is one of the main antioxidants in vegetables47, however, its content was similar in the previously described cases. The total antioxidant capacity in pulp determined as a percentage of inhibition of the radical DPPH was within the range obtained for B. capitata in four localities in the north of Minas Gerais, Brazil, 17.20 to 58.39%38. The total antioxidant capacity of a plant tissue depends on its chemical compounds and their concentration55)(62. The compounds measured in the butia fruits studied, showed a different contribution to the TAC in peel and pulp (Table 4). The TAC had a greater correlation with vitamin C in the peel than in the pulp, and, on the contrary, the correlation of the TAC with the TP and lutein was higher in the pulp than in the peel (Table 4). At the same time, the correlation of β-carotene with TAC was similar for the peel and the pulp (Table 4). In fruits and vegetables, TAC is part of a complex system that includes fat-soluble compounds (α-tocopherol, β-carotenes), water-soluble compounds (ascorbic acid and glutathione), antioxidant enzymes (peroxidase, superoxide dismutase, catalase) and other compounds such as isoflavonoids, phenols, polyamines and amino acids, such as cysteine and methionine55.
4. Conclusions
This study has allowed characterizing the peel and pulp of three colors of fruits of Butia odorata (Barb. Rodr.) Noblick by their physical attributes and chemical compounds of nutritional interest and by their antioxidant capacity. The butia peels studied had a higher content of provitamin A (β-carotene and β-cryptoxanthin), lutein, total polyphenols and dietary fiber than the pulp. This information can be useful, since it gives a differential value to the use of the peel as a by-product and, in particular, for its β-cryptoxanthin content; and the yellow fruits for their lutein content. Moreover, the three types of fruits would provide a similar amount of dietary fiber and vitamin C. In addition, intake of red and orange fruits whole would be an important source of carotenoids and polyphenols due to their content in the peel. When eaten whole in natura (peel and pulp), butias are an interesting complement to the diet due to their contribution of vitamin C, dietary fiber, provitamin A, lutein and total polyphenols.