1. Introduction
Despite the abundance of fruits in the world, there are a large number of native species in Brazil that are underexplored1. In particular, in south Brazil there are several native fruits with great potential for their use in the food and pharmaceutical industry2)(3. Among these, the Myrtaceae family stands out, with several commercial fruit trees2, as the cattley guava (Psidium cattleianum Sabine), Suriname cherry (Eugenia uniflora L.), guabirobeira (Campomanesia xanthocarpa Berg), guabijuzeiro (Myrcianthes pungens Berg) and uvalheira (Eugenia pyriformis Cambess)4.
These species have high economic potential because, in general, their fruits are berry type possessing desirable pulp yield and organoleptic characteristics, and nutritional and phytochemical aspects desirable for both fresh commercialization or the manufacture of innovative food products5.
These fruits also contain substances of nutritional and potentially functional importance. They present dietary fiber, antioxidant compounds, including phenolics and carotenoids, vitamins (especially A and C), and minerals (potassium, iron, manganese, magnesium, calcium, phosphorus)2)(3, offering an interesting option for special consumer markets interested in the presence of compounds potentially capable of preventing diseases6)(7.
The consumption of fruits and vegetables rich in bioactive compounds such as phenolics, carotenoids, terpenes and anthocyanins has the potential to prevent chronic non-communicable diseases, such as cancer, diabetes mellitus, dyslipidemia and cardiovascular and chronic respiratory diseases8.
In this review, summarized information on the main native fruits of the Myrtaceae is presented, highlighting their composition and biological activities in order to direct new research.
2. Physical-chemical and bioactive composition, and biological activity
2.1 Cattley guava (Psidium cattleianum Sabine)
Psidium cattleianum is known by different names in Portuguese, such as araçá, araçá-rosa, araçá-de-comer, araçá-da-praia or araçá-coroa, in English as cattley guava, strawberry guava or cherry guava, and in Spanish as arazá9. In Brazil, it is mostly known as araçá and its fruit is widely consumed fresh or processed into jams, jellies, ice creams and juices10.
The cattley guava skin can be yellow or red, and the yellowish white pulp is juicy, sweet, a little bit acid and spicy, containing multiple seeds9)(10.
Fresh fruits contain mainly water (81.7 - 84.9% w/w), followed by carbohydrates (4.3 - 10%), fiber (3.9 - 6.1%), protein (0.75 - 3.7%), minerals (0.63 - 1.50 g) and lipids (0.42-0.55%)9. The average total caloric content is 26.8 kcal/ 100 g of fresh fruit. The main minerals present in the pulp are calcium, magnesium, sodium, potassium and zinc9)(11. The variation among data is related to edaphoclimatic factors11 and quantification methods employed.
In addition, cattley guava fruits are a source of vitamin C, fatty acids, polysaccharides, volatile compounds, carotenoids and polyphenols9. Among the bioactive compounds, ellagic acid and its deoxyhexoside and epicatechin gallate are the main phenol, while all-trans-lutein, all-trans-antheraxanthin and all-trans-β-carotene are the main carotenoids12) in the literature (Table 1).
Table 1: Concentration of carotenoids and phenolic compounds from cattley guava fruits5)(13)(14)(15)(16
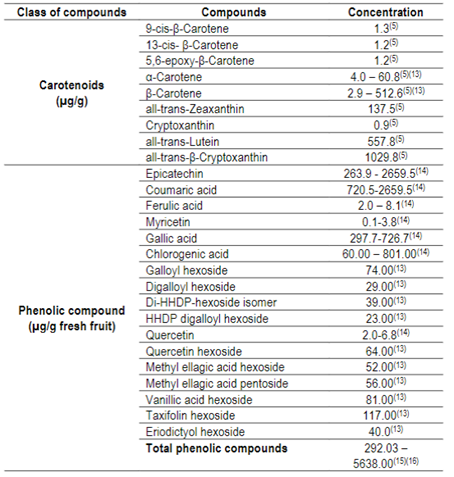
Cattley guava fruit extracts show high antioxidant activity12 able to neutralize free radicals, reducing the level of oxidative stress in the body, as well as preventing chronic non-communicable diseases17. In addition, they exhibit α-glucosidase inhibitory capacity after gastrointestinal digestion, indicating their potential use in the control of type II diabetes18. Antidiabetic, anticarcinogenic, antimicrobial, anti-inflammatory and anti-aging effect of Psidium cattleianum Sabine are attributed to its polyphenolic composition9)(14.
In vivo studies using rats fed with freeze-dried fruit cattley guava powder showed a remarkable reduction in the parameters altered by the oxidative stress induced by cisplatin. The animals showed reduced levels of glucose, LDL cholesterol, oxidized LDL cholesterol and total cholesterol when compared to the control animals (animals induced by cisplatin not fed with cattley guava)13. In addition, animals fed with cattley guava decreased the deposition of fat in the liver, showing an improvement in the lipid profile13. More recently, the administration of a cattley guava extract (200 mg / kg / day, for 21 days) in insulin-resistant rats prevented hepatic lipid peroxidation and the formation of reactive oxygen species avoiding hyperglycemia, hypertension and hypertriglyceridemia19. The effect of administration of a red cattley guava extract (200 mg / kg / day for 150 days) on the metabolic parameters of Wistar rats fed with a highly palatable diet also prevented changes caused by the diet, such as glucose intolerance, increased weight gain and visceral fat, high serum glucose levels, triacylglycerol, total cholesterol, LDL cholesterol and interleukin-620.
In none of these publications authors report the mechanisms of action related to the extracts. However, in the case of antihyperglycemic activity, it can be related to some of the phenolic compounds present in cattley guava extracts, since these compounds are able to inhibit specific enzymes, such as α-glucosidase and α-amylase21. Quercetin, for example, is considered a good inhibitor of digestive enzymes, with an IC50 almost 40 times lower than the positive control (acarbose)22.
Moreover, in Wistar rats with metabolic syndrome effects were observed of red cattley guava extracts on inflammatory and thrombo-regulatory parameters. The cattley guava extract (200 mg / kg / day for 150 days) prevented the increase of the following inflammatory parameters: interleukin-6 and butyryl-cholinesterase in serum, acetylcholinesterase in lymphocytes. Also preventing the decrease in thrombo-regulatory parameters: NTPDase activity in lymphocytes and platelets, and 50-nucleotidase in platelets23.
Cattley guava hydroalcoholic extracts (75:25 v/v; ethanol-water) showed antimicrobial potential against Staphylococcus aureus with minimum inhibitory concentration of 9 mg / mL and minimum bactericidal concentration of 18 mg / mL. In addition, the authors observed that the application of the extract caused a rupture in the cell membrane of the microorganism24. Extracts obtained by supercritical CO2 and cattley guava essential oil showed high total antioxidant activity and inhibitory effects on S. aureus and L. monocytogenes25.
Cattley guava fruit has high biological potential, due to its bioactivity, despite not being produced on a commercial scale. Nevertheless, there are several options for its industrialization. For example, red and yellow cattley guava jams were made without the addition of sugar, to promote healthier options for consumers. The results showed that the important bioactive compounds (phenolic compounds, anthocyanins and ascorbic acid) were not lost and these new products may be a good option for the use of cattley guava pulp26. The red cattley guava pulp was also encapsulated with excellent results in the retention of bioactive compounds and maintaining antioxidant activity27.
2.2 Suriname cherry (Eugenia uniflora L)
Suriname cherry (or Brazilian cherry) fruits are globular, crowned by persistent sections, with flattened poles presenting seven to ten ribs in the longitudinal direction, and with a slightly acidic and sweet taste28. Depending on the genotype, the epicarp varies from green to orange or light red at the beginning of ripening, turning to orange, red or dark purple (almost black) when they are fully ripe29.
Fresh Suriname cherry fruits differ in composition due to their color (genotype) and edaphoclimatic factors28. Yellow fruits have an average content of 84.7% (w/w) moisture, 10.3% carbohydrates, 1.1 to 3.7% protein, 2% fiber, 1.7% minerals and 0.02 to 0.5% lipids. In parallel, the red colored fruits consist of 83.9% (w/w) moisture, 13 to 19% carbohydrates, 0.8 to 5% proteins, 2.2% fiber, 1.1% minerals, and 0.4 to 0.9% lipids. Finally, purple fruits are composed by 81% (w/w) moisture, 14.8 to 19% carbohydrates, 1.2 to 5% protein, 2.4% minerals, and 0.4 to 0, 9% lipids30)(31)(32)(33.
The pulp of the Suriname cherry fruit has high levels of vitamin C34; the content is higher in orange, followed by purple and red fruits34. The fruits are also rich in vitamin A, carotenoids, anti-oxidant compounds with low lipid and caloric levels35)(36. Extracts of this fruit contain alkaloids, glycosides, flavonoids, tannins, saponins, terpenoids and reducing sugars, where flavonoids and glycosides are the main constituents37.
The high pulp yield (66 to 88% w/w of the fruit)35 allows both fresh as well as processed consumption (frozen, dehydrated and freeze-dried sweets, juices, ice creams, liquors or pulps)38.
This native fruit also has a high antioxidant potential, especially due to its composition of flavonoids, particularly myricetin and quercetin38)(39. It also contains carotenoids such as lycopene, γ-carotene and β-cryptoxanthin2)(29)(40. Table 2 shows the carotenoids and phenolic compounds contained in the Suriname cherry, according to the literature.
Table 2: Concentration of carotenoids and phenolic compounds from Suriname cherry29)(35)(38)(40)(41)(42)(43)(44)(45)(46)(47)(48
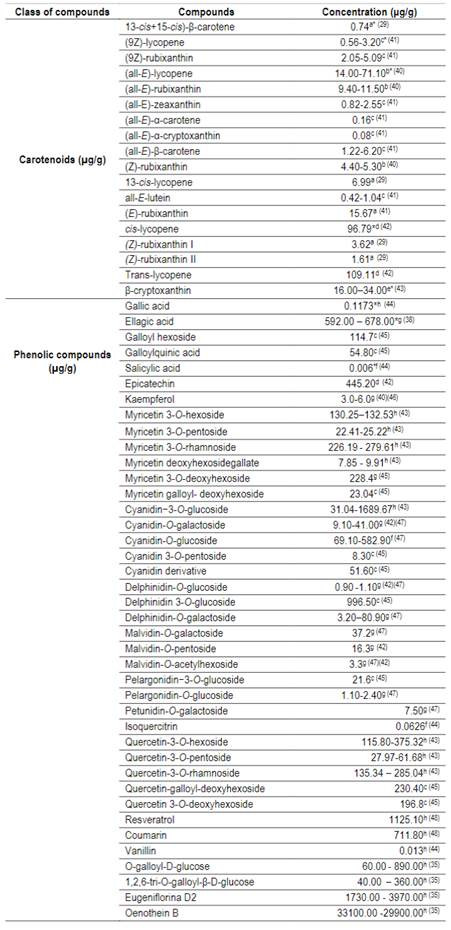
*afreeze dried pulp; *bfrozen pulp; *clyophilzed fruit; *djuice frozen; *efresh pulp; *flyophilized juice; *gfresh fruit; *hdried basis.
Purple, orange and red Suriname cherry have the capacity to eliminate free radicals34, suggesting that the consumption of this fruit may contribute to reduce the risk of developing chronic non-communicable diseases, recurrent of oxidative damage32. According to Vinholes and others22 the consumption of Suriname cherry contributes to the prevention of the development of type 2 diabetes. The purple extract was the most effective in inhibiting α-glucosidase, when compared to extracts of red and orange colors; and still, with an IC50 6 times lower than that of acarbose22.
Red Suriname cherry was investigated for possible activity in the prevention of obesity, and, consequently, in the metabolic syndrome49. It was demonstrated that in rats fed with a highly palatable diet the extract prevents the increase of visceral fat mass, glycemia, total cholesterol and LDL cholesterol. The extract was also able to prevent the reduction of the activity of the antioxidant enzymes superoxide dismutase and catalase. The results showed that Suriname cherry extract exerted anti-hyperglycemic, anti-hyperlipidemic and neuroprotective activities49. The same study also noted that Suriname cherry seed extracts have activity on sucrose and maltase enzymes, showing a possible antihyperglycemic effect50.
Another study showed that red Suriname cherry juice resulted in significant inhibition of acetylcholinesterase activity, a target enzyme in strategies for the treatment of Alzheimer's disease44.
Eugenia uniflora fruit extract showed a protective effect in mice with a depression model: prevented the depressant-like effect induced by chronic unpredictable stress; regulated the activity of acetylcholinesterase; reduced oxidative damage to lipids and reactive oxygen species production, in the prefrontal cortex and hippocampus; and prevented the reduction of glutathione peroxidase in the hippocampus of animals subjected to treatment51.
Researchers demonstrated the anti-inflammatory action on human gingival epithelial cells using compounds from the purple Suriname cherry juice52. This activity was tested on human oral cells after stimulation with the oral Gram-negative bacteria Porphyromonas gingivalis, which resulted in an 52% inhibition of the release of interleukin-8 stimulated by lipopolysaccharides. These results were associated with the presence of the cyanidin-3-glucoside and oxidoselina-1,3,7(11)-trien-8-one52.
Tambara and others45 investigated the effect of purple Suriname cherry extracts on the aging process, using the nematode Caenorhabditis elegans (model for the study of the molecular mechanisms of the aging process). The results showed that the ethanolic extract, rich in polyphenols, significantly increased the life span of C. elegans by modulating the DAF-16 pathway (a central regulator for various biological processes), without toxic effects. It also improved survival, reproduction and useful life in the pre-and post-exposure to oxidative stress45.
Ethanol extracts from Eugenia uniflora seeds also have antimicrobial potential against Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus and Salmonella Typhimurium. These activities were attributed to the polar compounds present in the seeds50.
Finally, the study by Becker and others53 demonstrated an antimicrobial effect of essential oil extracted from the Suriname cherry leaf against the bacteria Enterobacter aerogenes and Salmonella Typhimurium.
In brief, Suriname cherry fruit is a promising source of bioactive compounds that can reduce or reverse the damage caused by reactive oxygen species due to its high antioxidant activity, demonstrating its potential for pharmaceutical and food purposes.
2.3 Guabiroba (Campomanesia xanthocarpa Berg)
Guabiroba is a yellow-orange fruit with acid and sweet flavor, which can be consumed fresh or processed as jelly, ice cream, liquor or tea7. The fresh fruits of guabiroba have a moisture content varying between 79-83% (w/w), 8-16% carbohydrates, 6 to 10% fiber, 1 to 5.5% proteins, and 0.1 to 3.7% lipids. Thus, as in other native fruits, the composition is directly affected by edaphoclimatic factors5)(54.
The fruit contains macro and micronutrients: proteins (1.08 g / 100 g), fats (0.12 g / 100 g), reduced sugar (8.3 g / 100 g, being fructose 4.0 g / 100 g; glucose 4.3 g / 100 g), mineral salts (iron 3.52, calcium 28.45, phosphorus 25.3 mg / 100 g, potassium 1.53 mg / 100 g)54.
This fruit stands out for its content of vitamin C, minerals, vitamin A, and it is considered rich in fiber55, with a high content of soluble fiber, mainly pectin56, and also in phenolic compounds, including chlorogenic acid, and carotenoids with high antioxidant potential5)(57)(58.
Anthocyanins, such as cyanidin-3-O-glucoside, delfinidin-3-O-glucoside and pelargonidin-3,5-diglucoside59, have already been identified in guabiroba fruits, as well as cryptoxanthine, lutein and β-carotene5)(60, the latter representing more than 40% of the total of these bioactive compounds55. The individual carotenoids and phenolic compounds reported in the literature are presented in Table 3.
Table 3: Concentration of carotenoids and phenolic compounds from guabiroba5)(47)(54)(55)(59)(60)(61)(62)(63
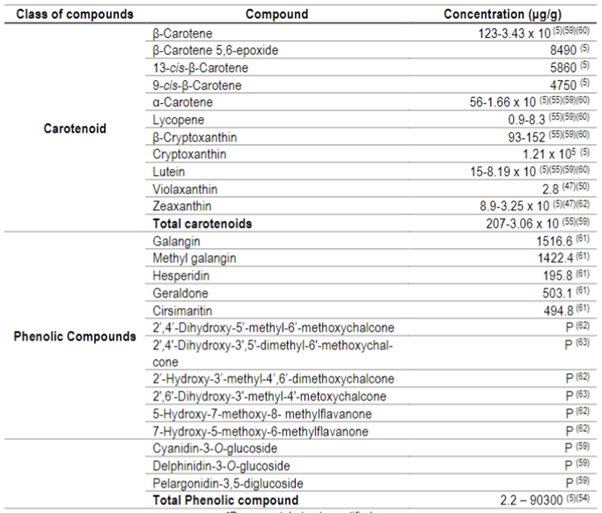
*P= present, but not quantified.
The biological effects of guabiroba consist of antiproliferative, anti-obesity, hypoglycemic and hypolipidemic activities7. The compound 5-hydroxy-7-methoxy-8-methylflavanone isolated from guabiroba extracts proved to be effective against melanoma, breast, ovarian, kidney, lung, prostate and colon cells, without being toxic to vero cells (normal cell), showing antiproliferative activity of these extracts62.
Hypoglycemic activity was observed in obese rats (induced with a high-calorie diet), where a 15% reduction in the plasma glucose level of the animals was observed64.
Acetylsalicylic acid is widely used as a treatment for the prevention of atherosclerotic disease; however, it can produce hemorrhagic events, including gastric ulcers65. Guabiroba extracts have been compared to acetylsalicylic acid, proving to be safer than the drug against stomach injuries, with a positive effect on improving blood circulation66.
Regginato and others67 evaluated the antihyperglycemic and hypolipidemic effects of guabiroba seed extract in hyperglycemic rats, observing that the application of the extract in concentrations of 400 mg / kg promoted a decrease in blood glucose levels in the animals, as well as an increase in muscle and liver glycogen, and reduced enzyme activities of saccharose and maltase. In addition, the same dose of extract decreased serum levels of LDL cholesterol, while the application of the 200 mg / kg dose promoted an increase in HDL cholesterol levels67.
The use of guabiroba in the formulation of new products is encouraged, but it is important that the compounds remain preserved60. Santos and others60 found that the content of carotenoids that remain in jellies after fruit industrialization is maintained at up to 30% of the initial concentration, being quite promising as functional food. In addition, the encapsulation of guabiroba pulp with Poly (d,l-lactic-co-glycolic) acid reduced the generation of reactive oxygen species in non-cancer cells68.
2.4 Guabiju (Myrcianthes pungens Berg)
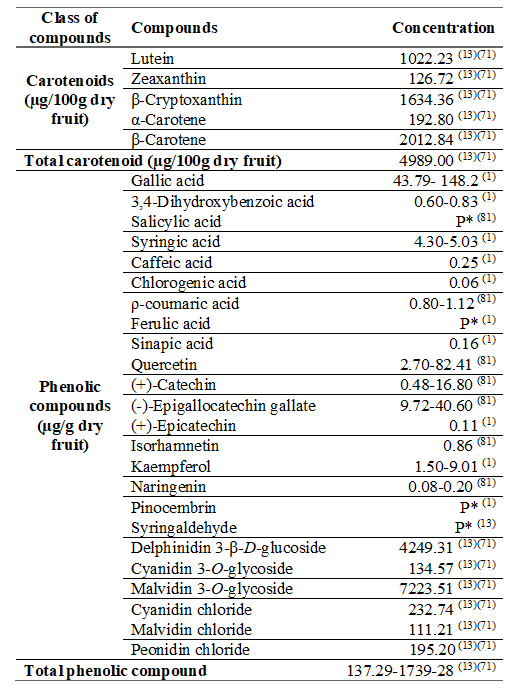
Guabiju is a spherical and velvety fruit, purplish when maturity, succulent and with yellowish and edible pulp. It has an astringent flavor (with more or less pungency) when ripe, and sweet4)(69. Fruits contain a maximum of two seeds of 6 to 7 mm, which are smooth and have a thin coat70)(71. The peel is thick persistent calyx and changes from brownish green to dark purple during ripening1)(13)(71)(72.
The fruit is highly perishable, with a maximum shelf life of 3 days73, because it is extremely fragile in structure, requiring special care in the harvest4. Freeze-drying or freezing the pulp are options for its conservation that enable the maintenance of most bioactive compounds74, especially anthocyanins, phenolic compounds and carotenoids75. The concentration of anthocyanins increases during storage at room temperature (25 ºC)75.
As for the nutritional composition, the guabiju hard fruits have a moisture content ranging from 81 to 85% (w/w). Evaluating the other compounds on a dry basis, fruits have 4.9 to 64.6% (w/w) of carbohydrates, 4.4 to 32.4% of fiber, 0.6 to 8.4 % of proteins, and 0.3 to 0.8 % lipids13)(73)(76.
Fruits are rich in phenolic compounds77)(80)(81) especially anthocyanins and flavonoids, responsible for the purplish color of the bark, containing also carotenoids, mainly in the pulp82.
Among the phenolic compounds in guabiju gallic acid, isoquercitrin, hiperoside, quercetin, quercitrin, and delphinidin 3-β-D-glucoside were found; and as anthocyanins, cyanidin 3-O-glycoside, malvidin-3-O-glycoside, cyanidin chloride, malvidin chloride and peonidin chloride. Malvidin 3-O-glycoside was the main anthocyanin found in the fruit76.
Some carotenoids are also found in the pulp and bark of guabiju, such as lutein, zeaxanthin, β-cryptoxanthin, α and β-Carotene69. Table 4 shows the carotenoids and individual phenolic compounds found in guabiju fruits.
Studies demonstrate guabiju high antioxidant potential78, which increases during its maturation80. This potential is closely linked to the gastroprotective76 and anti-cholesterolemic effects13 contributing to the prevention and recovery from chronic diseases. Studies relate the phytochemicals of the fruit to anticholinesterase78 and anti-chemotactic effects82)(83)(84.
Gastroprotective activity has already been linked to the ingestion of guabiju fruit extracts. Animal models with an acute ulcer received methanolic extracts of bark, pulp and leaves, and showed a defense capacity of the gastric mucosa, reducing the relative area of the lesion when compared to the control76.
A qualitative study with extracts of guabiju showed that the extracts inhibit the enzyme acetylcholinesterase having an action similar to the medications used in the treatment of symptoms of Alzheimer's disease79. An in vivo study of guabiju fruits showed a protective effect against the oxidative stress caused by cisplatin in animals that consumed a diet that included the fruits. The levels of total cholesterol, low-density lipoproteins (LDL) and oxidized low-density lipoprotein (Ox-LDL) in the plasma and fat in the liver decreased without increasing body weight after consumption of the fruit diet71.
The extracts of guabiju leaves have been shown to be effective against Strongyloides venezuelensis and may be a possible drug against strongyloidiasis85, a silent, underdiagnosed and very neglected helminth disease86.
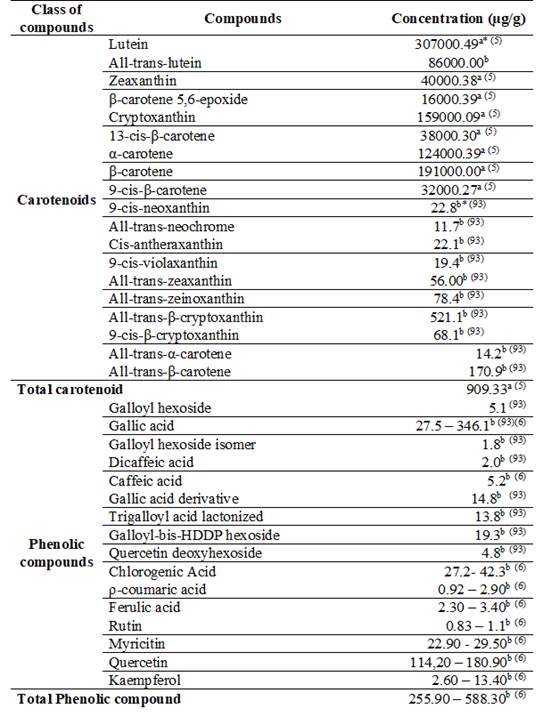
2.5 Uvaia (Eugenia pyriformis)
The uvaia fruit is rounded, 2 to 4 cm in diameter, with a very thin skin, slightly velvety and yellow gold in color, with orange ripeness. The pulp has a succulent, sweet, acidic and aromatic flavor78 and can be consumed fresh78)(87)(88. However, it is an extremely perishable fruit and an interesting alternative for processing it in the form of jelly, yogurt, ice cream, frozen pulps, juices and jams79)(87)(88)(89, in addition to wines and vinegars87)(88). The pulp is rich in vitamin C and contains considerable amounts of vitamin A and the B complex5)(59. Despite this, and because it is often acid, fruits are usually consumed in the form of juice, jelly, liqueurs, jam, ice cream and sweets90.
Fresh fruits contain, on average, 90% (w/w) water91)(92. When evaluating fruits on a dry basis, an average of 44% carbohydrates, 42% fiber, 5.5% minerals, 5.5% proteins and 2.2% lipids are found87. The fruit is also rich in calcium, magnesium, potassium and iron59)(87)(91)(93.
Uvaia fruit has higher antioxidant activity (3246 g/g DPPH) than mangaba (3385 g/g DPPH) and açaí (3778 g/g DPPH)94.
The phytochemicals present in the fruit are phenolic acids, flavonoids and carotenoids2. The main carotenoid parents found by Pereira and others5 were: lutein, zeaxanthin, α and β-carotene. The seed has phenolics, flavonoids and high antioxidant activity91. The concentration of carotenoids and individual phenolic compounds is shown in Table 5.
The residue from the processing of ground uvaia pulp (peels and seeds) is an appropriate and promising alternative as a natural color in confectionery (sugar hard-panning confections)95, and has antileishmanial antifungal and antiproliferative activities96.
In an in vivo study, the administration of uvaia juice in the treatment group caused a significant reduction in the concentration of protein carbonyls in the rat liver (47.4%), which was associated with a 29% increase in catalase activity in addition to other biochemical parameters, demonstrating that this native fruit reduces oxidative damage, improves antioxidant efficiency and attenuates the oxidative damage to proteins caused by free radicals97. In addition, the extract showed anti-chemotactic, anti-inflammatory and antioxidant activities98.
The study by Stieven and others98 found that the essential oil obtained from uvaia has antimicrobial activity against Staphylococcus aureus and Escherichia coli.
4. Conclusions
Brazil has a wide variety of native fruits, still unexplored, with high potential for commercial and/or industrial use. The native fruits mentioned in this review should be stimulated for consumption given their high nutritional value, rich contents of vitamins and minerals, and especially of phenolic compounds and carotenoids. Moreover, diseases such as diabetes, cardiovascular diseases and some types of cancer could be avoided through the ingestion of these natives, showing that their use should be stimulated.