1. Introduction
Cochliomyia hominivorax (Diptera: Calliphoridae), the New World Screwworm (NWS) fly, ‘mosca de la bichera’ or simply ‘bichera’ as known in Uruguay and the region, is an obligatory ectoparasite that causes myiasis in warm-blooded vertebrates, including humans1. Myiasis, as defined by Zumpt2, is the ‘infestation of live animals by dipteran larvae that at least during some developmental phase feed on host’s flesh and fluids’.
The first record of the species was in 1858 by C. Coquerel, a French medical doctor and entomologist who collected larvae from the frontal sinuses and nostrils of a man held in the Devil’s Island penal of Cayenne, French Guiana. Originally, he named the species Lucilia hominivorax, denoting its striking characteristic, as hominivorax is roughly translated as ‘man-eater’. His report in the Annals of the Entomological Society of France3 described several human cases with high mortality. Coquerel’s article went unnoticed until the 1930’s decade and for example the NWS fly was confused with Cochliomyia macellaria in the Americas, a scavenger of carcasses. This misidentification was solved by Cushing and Patton4, which analysed genitalia of the flies and named the parasitic species Cochliomyia americana. Later it was found to be the same species described by Coquerel 75 years earlier and was finally named Cochliomyia hominivorax5.
The following review provides an integrative overview of the epidemiological and economic impact of myiasis, and discusses the NWS fly control in Uruguay and region as the likely solution going through the history of sterile insect technique (SIT)-based and novel biotechnology-based strategies.
1.1. Life cycle
The NWS life cycle involves a complete metamorphosis (eggs, three larvae instars, pupae and fly). The pupae and adults make up the free-living phase which is critically influenced by environmental conditions. On the other hand, eggs and larvae develop under host temperature. Its cycle spans for 24 to 60 days, depending mostly on the temperature and humidity1)(4)(6)(7. Adult gravid females lay their eggs on the dried edges of wounds and bodily orifices of animal hosts. Between 12 to 24 hours later the larvae hatch and begin feeding from the animal’s flesh and fluids5)(8. Larvae go through three stages of instar (L1, L2 and L3), for about 4 to 8 days after which L3 larvae matures and leaves the wound. As it falls it screws into the ground to pupate5)(9 and later adults emerge. Time between the falling of the larvae and the emergence of the adult depends on environmental and climatic conditions. After 24 hours of emergence males are sexually mature and are polygamous, mating 5-6 times during their life10. On the other hand, NWS fly females are monogamous11, able for mating two to five days after emerging. They are autogenous (i.e., females can produce eggs without a first protein meal) at least for the first reproductive cycle, being ready for oviposition at least four days after mating12.
1.2. Geographic distribution, habitat preferences, seasonality and abundance
The NWS fly is a tropical-subtropical species endemic to the Americas, present in almost all biomes since long time ago. A phylogeographic analysis including NWS samples from South and Central America, and the Caribbean support the hypothesis of a North to South spread that must had begun during the Pleistocene, and indicates it must had reached its current geographical range during the Holocene13. The NWS demographic history as revealed by mtDNA sequences supports that the human settlement of the Americas modified its habitat by introducing a novel host that could have enlarged the fly populations, a process reinforced by livestock introduction 500 years ago13. The screwworm prefers warm humid climate and is not homogeneously distributed14, it is mainly associated with water courses15 inhabiting the ecotone between forests and grasslands, and semi-open forests16)(17)(18)(19. The Pampas biome is dominated by grasslands and riverine forests, which during the spring-summer season with warmer temperature constitute an ideal habitat for the NWS fly. In addition, the hillside forests and wetlands complement this favourable environmental setting. Another factor that could support the NWS fly development in the region is the landscape modification introduced by the current forestation intensification. Naturally, its abundance increases due to warm-blooded animal density14, something extremely high, because of livestock, in the Pampas biome. There are also high abundance differences between sites within the same habitat, with a split among feeding, mating and oviposition sites18)(20, indicating its sensitivity to micro-environmental variations. Field observations and artificial habitat reproduction indicate that newly emerged individuals fly out the forest seeking for food and rest, preferring flowering trees21)(22. Mark-release-recapture data have also shown that NWS females have preferences for forests, but they fly in nearby grasslands looking for hosts to lay eggs23.
Historically, the NWS fly was geographically distributed from the southern United States to central Argentina, including the Caribbean islands24. The Edwards Plateau in Texas was indicated as its northernmost limit, but registered up to north Iowa (43° parallel) in the warmer months every year25)(26. The southernmost limit was indicated at the 45° parallel, Province of Chubut, Argentina27. The central region of Argentina, like Uruguay, is a transitional zone where the NWS fly behaves seasonally, and at the north of parallel 29° is year-round present28. Mean winter temperature below 9°C is considered a threshold determining the NWS fly distribution10)(25)(29)(30)(31 and emergence of adults is ~1% at -6°C32. Its high dispersal ability shapes its distribution in the north hemisphere extending its range northward from overwintering subtropical areas32)(33. The main hypothesis is that a small propagule with few individuals can re-invade ‘marginal’ areas when conditions favor its survival and reproduction return34)(35.
Many authors evaluated its flight capacity, Barrett25 indicates that a NWS adult fly can move 56 km in its life, whereas Mayer and Aztemi36 estimated a dispersal rate of 3 km/day when conditions are favorable, and finally Bush and others37 estimated that most adults fly around 16 km/day, but some outliers flying 290 km were reported. More recently, in an experiment using marked flies conducted between Argentina and Uruguay in order to test the Uruguay River as a natural barrier to dispersal, individuals were recovered at 13.9 km and 15 km from the releasing point, respectively7. Despite its great flight ability and high reproductive potential, the NWS fly density is relatively low, around 200 flies/km2(37)(38. More recent studies carried out in a tropical region estimated between 10 to 120 flies/km2 and a dispersion that did not exceed 7 km39, indicating that it is able to double the population in about 100 days40. However, the relationship between its abundance and the climate has been difficult to establish; some studies did not find a significant correlation41)(42 but others did43)(44. Parman32 suggested that drier soils benefit pupae emergence in winter, while humid soils reduce pupae viability at any time of the year. But Thomas45)(46 reported a high resistance of pupae to drowning, and did not find negative effects of moisture on adult emergence in southern Mexico. On the opposite side, mortality of mature larvae, pupae and adults has been reported as high due to desiccation47)(48. In Argentina and Uruguay, as expected, the mortality of larvae and pupae is higher during winter7)(28. In the central region of Argentina, it was shown that adult hatching percentage increases with temperature (from 47% at 14°C to 97% at 24°C), and flooding generate the death of pupae due to lack of oxygenation49. In Uruguay, no adult emergence was registered during the severe winter of 2016, and the first emergence was registered in spring (after 33 days of pupation) supporting a seasonal behaviour, but fertile adults were recovered during the less severe winter of 2017. This emergence rate difference was also evident between the north and south of the country, revealing the temperature influence on the species occurrence, emergence and pupal period span7.
In order to investigate the role of weather in the eradication of the NWS fly, Gutierrez and Ponti50 parameterized a physiologically based demographic model using public data on developmental times, fecundity and mortality rates on temperature to characterize its year-round persistence range. They showed a strong influence of winter temperature and rainfall on NWS outbreaks in Texas, USA, from 1962 to 1982, and Libya, from 1988 to 1992, and determined that the optimal temperature to complete the life cycle is 27.2°C, also determining that the putative lower and upper thermal thresholds are 14.5 and 43.5°C, respectively.
In summary, warm and humid climates have been associated with NWS fly abundance, and dry climates, both cold and hot, with low abundance32)(51)(52)(53)(54)(55. This notion that climate governs NWS fly abundance is implicit in the seasonal nature of myiasis in domestic animals51. To our knowledge, no study is evaluating how the current warming will modify the NWS fly distribution and dynamics, if southern South America will be colonized and/or will cause myiasis above a problematic threshold along the year in seasonally regions like Uruguay.
1.3. Genetics and population genetics
The NWS fly genetics have been under active investigation, from cytogenetics to molecular and population genetics unlocking many aspects of its biology, as well as generating molecular tools extremely useful for the development of new control strategies.
Cytogenetic maps allow physical and genetic maps to be integrated, becoming an invaluable tool for the genetic analysis and manipulation of any species. The NWS fly has a diploid number of 12 chromosomes (2n = 12), with five pairs of autosomes and a pair of sex chromosomes (XX for females and XY for males)56. Polytene chromosome photomaps of the NWS fly have been described57)(58, and more recently updated with a resolution of 1450 bands59. The genomics era of this pest species began with the mitochondrial genome sequencing60 and recently the whole nuclear genome sequence was described61. A major advantage of genomics data is the opportunity to deeply address questions relating to taxonomy and systematics, molecular evolution, population divergence, gene function and adaptation. An example of real time evolution, extremely relevant for control programs, is insecticide resistance. To investigate this in the NWS fly a transcriptomic approach has been used to measure metabolic detoxification enzyme families (i.e., cytochrome P450 monooxygenases, glutathione S-transferases and carboxyl/cholinesterases)62 and identified mutations in genes related with organophosphates and pyrethroids insensitivity63)(64)(65)(66)(67.
Population genetics is essential in fields like evolution, systematics, ecology, conservation and wildlife management to understand the causes of genetic differences within and among species. In pest management, population genetics will allow through the inference of divergence and migration rates to quantify the degree of geographic or ecological isolation among regions, useful information to define the target area and scale of a control program. The genetic variability and population structure of the NWS fly have been characterized using cytogenetic markers66)(68)(69)(70)(71)(72, isozymes73)(74)(75)(76)(77)(78, RAPDs79, RFLP, PCR-RFLP and sequences of mtDNA13)(80)(81)(82)(83)(84)(85)(86)(87)(88)(89, microsatellites90)(91)(92, and SNPs93. These 40 years of research support that NWS fly is a single highly polymorphic species.
On the continental-wide scale, the NWS fly is structured into four regional groups of populations: CG (Cuba), DRG (Dominican Republic), NAG (North Amazon Group: Jamaica, Trinidad and Tobago, Colombia, Ecuador and Venezuela) and SAG (South Amazon Group: Brazil, Paraguay, Uruguay and Argentina)87. Population divergence models and historical demography based on mtDNA sequences support a population expansion process that has started in the north, with a first split between North/Central America and South America populations after the Last Glacial Maximum (15,300-19,000 YBP), followed by a second split between NAG and SAG in the Amazon region during Pleistocene and Holocene (9,100-11,000 YBP)13. Interestingly, NAG and SAG do not share mitochondrial haplotypes indicating a high degree of isolation between the northern and southern regions of South America13, despite connection corridors were predicted in the Atlantic Ocean coast region and through the Northwest Brazil and Peru94. This geographic structure has been interpreted as due to a barrier at the north of the Amazon basin, although not yet described88. SAG has low population differentiation without geographic structure for mtDNA87)(89, probably due to population expansion process13. Within SAG, populations from Uruguay were described as a single panmictic population based on mtDNA data84 or with low divergence based on nuclear microsatellites90.
More recently, protocols for specific gene disruption using the Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR) and the CRISPR-associated protein 9 (Cas9) technology (CRISPR/Cas9)95 have been successfully developed in the NWS fly96)(97. This functional genomics approach opens a new avenue to study gene function in vivo and for the development of biotechnological based strategies for insect pest control.
2. Parasitology and epidemiology
As described, the NWS life cycle consists of two phases: the parasitic phase, that involves the three larvae stages which extend for a fairly constant period of time as it develops in the host, and the free living phase, that involves the mature 3rd instar larvae which screws into the ground to pupate and after a complete metamorphosis emerge as adult flies. This last phase can be completed in eight days in optimal environmental conditions. After mating, through sexual reproduction, females lay eggs at wound edges or host orifices repeating its life cycle.
2.1 Parasitic phase
Although female flies are attracted to wounds of warm-blooded animals to oviposit, they may also do so on different body openings such as nostrils and vagina10, taking on average 15 minutes to lay their eggs10. A single female can lay around 200 eggs (ranging from 10 to 500) in an average of four oviposition, and as myiasis odour is attractive to other flies (i.e. facultative opportunistic blowflies), it results in infestations of hundreds or thousands of larvae, which causes host’s weight decrease, pain, agony and suffering for several days, and eventually death if not treated10)(23. The eggs laid by NWS female flies hatch between 12 and 24 hours. The hatching larvae (L1) tend to aggregate on wounds borders1) and feeding on the host’s flesh screwing their way into it leaving their spiracles facing the cavity opening in order to breathe10. They molt to second stage larvae (L2) 2 days after egg eclosion, and during this stage larvae gain some weight reaching 4.9 mg1. On the third day from infestation larvae molt to their third stage (L3) reaching 16 mg, keep growing until approximately 120 mg of weight while enlarging the injury they create1. During the last development stage, larvae produce an exudate which promotes bacterial infections and prevents the wound from healing. After 8 days from infestation almost all larvae at the third instar have left the wound falling on the ground to start the non-parasitic phase1)(10.
Despite the specific requirement of living tissues from warm-blooded animals for its parasitic phase, the NWS fly is extremely versatile considering the diversity of hosts where it can complete its development. This wide host range, from wildlife to domestic animals and humans, as well as where it can oviposit, including all kinds of wounds, even a tick bite, and bodily orifices illustrate its high consequence parasitic ability.
2.2. Free living phase
Once mature, L3 larvae reach the ground, screws into it to pupate, and after a variable period of days -depending on environment and mainly on soil conditions- they complete the metamorphosis to the adult form. It has been described that L3 larvae can screw into depths from 0.5 to 6 cm7)(9. So far it has been established that the depth to which L3 larvae can reach to pupate may vary due to soil type9)(10, ground vegetal cover10 and environmental temperature32. Although high moisture soils favour pupae survival, flooded grounds diminish it41. Warm seasons with higher temperatures shorten the pupation span7)(10, but persistent extremely high temperatures negatively impact pupae survival41. Therefore, the whole NWS life cycle is usually complete within 18 days at 29°C or 24 days at 22°C, and although the screwworm lacks a dormant overwintering stage (i.e. diapause), it can even extend the pupae phase for more than 50 days when facing harsh environmental conditions1)(4)(6. For instance, recent experiments conducted in Uruguay recovered viable flies after 57 days of pupation7.
Flies normally emerge early in the morning, and usually females show up first. Initially, the flies’ cuticle looks pale and soft, and their wings wrinkled and folded. After reaching the surface, they do not move for 15-20 minutes, while spreading their wings98. Some hours later, their exoskeleton hardens and gains a deep blue to bluish metallic colour, having on their thorax three perceptible black stripes, with the one in the middle shorter than the ones on the sides10. Males reach sexual maturity at 24 hours from emergence and are polygamous10. Females accept the male between two to five days after emergence1)(10)(99, and copulation generally occurs during daylight period and lasts between 1.6 to 3.8 minutes. The oviposition occurs between four to six days after copulation, when NWS females feels strongly attracted to animal’s wounds and start searching for a host to discharge their eggs1)(10)(99)(100.
2.3. Livestock
The screwworm was a chronic problem in southern USA26)(31)(101. Only in Texas, during 1935, there were 3.2 million NWS-related myiasis cases in cattle, of which about 15% of infested animals died102. Currently, NWS infestations remain a serious problem for animal health in South America. Costa-Junior and others103 reviewed the literature on occurrence of both larvae (myiasis-causing development stage) and adults in Brazil concluding that NWS fly is the most important cause of primary myiasis in livestock, pets and human beings, and it is distributed throughout the country. Other epidemiological reports in South America have shown the prevalence and incidence of the NWS fly, indicating its importance in a tropical area in Ecuador104, and have revealed that is widespread in Venezuela representing a serious threat to the livestock industry, as well as to the human population55.
In Uruguay, the NWS fly has been reported in the whole territory and it is difficult, if not impossible, to find a livestock farm without myiasis during spring-summer-autumn. This epidemiological situation makes the systematic investigation of the NWS fly prevalence necessary, as well as its population dynamics, in order to fine-tune any control efforts. A survey of the larvae from naturally infested wounds in ruminants (i.e. sheep and bovines) conducted in Uruguay, from November 1985 to May 1988, showed that nearly 88% were identified as C. hominivorax, while the remainder were assigned to Chrysomyia albiceps and Cochliomyia macellaria, both facultative myiasis-causing blowflies. The main injuries were observed in sheep hooves (~27%) and navels in new-born calves (~7%), and the authors highlighted the high incidence of myiasis on foot diseases of sheep during the investigation period. Interestingly, three myiasis with only larvae from secondary species (C. macellaria (n=2), C. albiceps (n=1)) were registered in this survey105. Another survey conducted from January to May 1988 in Uruguay, based on a questionnaire to livestock producers, indicated that myiasis was reported in all the surveyed farms and the prevalence was 4.5% in cattle and 6.2% in sheep54. Also, the Official Veterinary Services of Uruguay (DGSG) has made numerous efforts to determine the impact of NWS. In 2006, a survey was carried out using the 2005 national livestock database (DICOSE) as the sampling frame106. A total of 150 from the 49.431 registered Uruguayan farms were selected by simple random sampling according to each of four strata of production type: sheep (predominantly), mixed (beef cattle and sheep), beef, and dairy farms. Data was collected through personal interviews with the livestock owners between July and November 2006. The myiasis estimated prevalence was 3.4% in cattle and 5.7% in sheep, in close agreement with the previous report, whereas mortality was 0.06% in cattle and 1.25% in sheep. It is remarkable that livestock owners declared to plan management practices, such as cattle castration and sheep docking, shearing and births, when the presence of the NWS fly is low. More recently, from August to November 2014, DGSG performed a new survey using DICOSE 2013 to select 650 from the 45.059 total farms by a stratified random sampling of three strat1. Again, data was collected through personal interviews and showed that 76.6% of livestock owners plan the management practices trying to avoid the NWS fly season. The myiasis estimated prevalence was 3.7% in cattle and 8.4% in sheep, whereas mortality was 0.1% in cattle and 2.5% in sheep. The three surveys agree to indicate a pronounced seasonal variation for NWS presence, being at its minimum during winter and at its maximum in the summer, as expected. Despite the methodological differences in each survey, a trend in the prevalence for sheep and cattle is observed at national level.
The myiasis occurrence was also investigated at a narrowed scale in the Department of Artigas (North of Uruguay, 30 °S), between August 2014 and April 2015107. In this survey a total of 164 livestock farms (9%), occupying 184,826 hectares (16%), with 339,227 livestock (132,877 cattle and 206,350 sheep, being 16% and 19%, respectively) were analysed. Sheep (69%) and cattle (30%) were the most affected, whereas, horses (0.5%) and pigs (0.5%) were barely affected. Within sheep, adult females (39%), and within cattle, calves (69%) were the most affected. Hooves’ myiasis were the most frequent in sheep, and calves’ navel myiasis were the most frequent in cattle. Interestingly, in this survey myiasis cases were registered during winter in the northernmost Uruguayan region. Based on livestock farms distribution, it was possible to hypothesize a link between myiasis occurrence and the landscape. Myiasis cases were higher when animals graze in riverine fields, near natural forest. Also, it was possible to establish a connection between livestock management activities, such as cattle identification with earrings, brand fire, castration, dehorning, shearing, sheep marking, etc., and the occurrence of myiasis. The traditional sheep shearing and cattle castration are the most important activities associated with myiasis occurrence.
In order to reliably determine the occurrence and local scale distribution of NWS myiasis during winter, a larvae sampling was performed from June 21 to September 19, 2015107. Nine livestock farms were selected in Artigas, each with a veterinarian responsible for larvae collection and cases reported. Larvae identification was done by morphological characters (bands of spines, spikes of spines, segments without spines, posterior spiracles, and tracheal trunks) following a key10. A total of 103 myiasis were recorded, from which 101 (98%) identified larvae were C. hominivorax, and 2 (2%) were C. albiceps. Almost 80% of myiasis were registered in three farms, one in the locality of Sepultura in a spiny savanna and shrubland with natural forest. Myiasis cases were recorded in 11 of the 13 weeks covered, with higher occurrences at weeks 2 and 10, and no cases only during weeks 7 and 13. Sheep were the most affected (88%) and all identified larvae were NWS, while remaining cases were in cattle. In this study, the authors reported that 66% of sheep myiasis were due to lambs’ tail docking, 21% in hooves possibly initiated in wounds generated by foot rot, 9% in vagina probably caused by injuries from lambing, and the remaining 4% were in navels, heads and necks. Cattle myiasis were mostly in brand wounds (33%) but only half of those were caused by NWS. Screwworm-related myiasis were uniformly distributed in brand, navel, vagina, ear, scrotum and other body wounds related to usual field management activities (i.e., fire branding, lambing and castration, etc.).
2.4. Wildlife
The NWS fly also affects wildlife warm-blooded vertebrates, but its incidence and impact are poorly documented. It has been indicated that prior eradication in the USA, around 2-3% of wild animals could be infested in endemic regions108. Well documented examples are the die-offs associated with myiasis of the white-tailed deer (Odocoileus virginianus texanus) in parts of the USA with fawns deaths ranging from 25% to up 80% depending on year conditions109)(110)(111, and myiasis reported in feral swine (Sus scrofa) in Florida (USA) in the 1950s, where the control of swine populations was considered a priority to reduce the NWS fly incidence in deer herds112. More recently, the importance of wildlife as NWS fly host was highlighted during Florida Keys (USA) outbreak in 2016, which resulted in the death of 135 endangered Key deer (Odocoileus virginianus clavium)113. In 2021, a total of 27 NWS myiasis in feral swine were reported in the north of Uruguay114. Therefore, myiasis surveillance in wildlife could enhance the efficiency of area-wide NWS fly management programs115.
2.5. Human cases
Myiasis in humans is neglected116 and under-reported, mainly due to negative social implications. Every human is a potential host, but those who cannot take care of themselves are especially vulnerable, like children, elders and mentally challenged persons. People living in NWS favorable areas, like the rural population of South America, are at a higher risk. In Uruguay it is not a notifiable disease and mostly outpatient treatments, so its real incidence is not known with exactitude. DGSG in its 2006 survey reported that 0.7% of the rural population is affected by NWS annually, corresponding to 818 human cases per year. But also other authors117)(118)(119 have been reporting human cases in urban areas, affecting children and adults. Myiasis were predominantly in the scalp and oropharyngeal region, and all cases were associated with pre-existing injuries and concomitant conditions, such as mental disorders, poor hygiene or alcoholism, among others. A recent study described the clinical and epidemiological characteristics of 63 myiasis cases in children (7 years old in average) hospitalized in a referral center in Uruguay between 2010 and 2019. It was shown that the vast majority of myiasis (98%) were caused by the NWS fly and one third of the patients presented comorbidities, with chronic malnutrition being the most frequent. About half of the cases were initiated in injuries caused by pediculosis120.
3. Economic impact
Several evaluations of the economic losses caused by the NWS fly have been conducted in Central and North American countries, and in Libya, where the favorable result of cost-benefit analysis (CBA) encouraged the implementation of eradication programs10)(121)(122)(123)(124. Many of these studies implemented surveys of farmers that made it possible to approximate the costs of the NWS fly per head of cattle. These studies could estimate the NWS fly impact on total production costs and the level of production per farmer by extrapolating the average per head cost to the entire herd. As stated by Wyss122, the annual benefits of NWS eradication for the American, Mexican and Central American farmers were estimated in 1999 at USD 870, 319 and 85.1 million respectively (USD 1,350, 495 and 132 million in 2020, adjusted for inflation). All affected countries reported that the most important direct losses are in manpower -rural working hours dedicated to myiasis control (and diverted from other productive activities)-, followed by animal deaths (mainly sheep), productivity decrease, medicines and veterinary supplies costs. Based on the previous estimates done by Wyss122 and Vargas-Terán and others123, the Mercosur farmers could save between USD 4,200 million and USD 4,760 million each year (values adjusted by inflation to 2020).
In Uruguay the livestock industry is a key sector representing more than 20% of goods exports, 17% of total employment and covering more than 70% of the country’s agricultural area125)(126. Quantification of NWS economic losses in Uruguay is a difficult task that has been approached through several studies (Figure 1), despite great intra- and interannual variability depending on climate conditions. Direct losses for livestock producers ranged from USD 40 million to 154 million annually adjusted to 2020 (i.e. between 2% and 8% of livestock gross domestic product, GDP). In a first attempt, the DGSG estimated in 1998 annual losses of USD 25 million (USD 40 million in 2020), distributed in: rural work (60%), veterinary supplies (8%), sheep (20%) and cattle (12%) mortality127. Later, set up on the second DGSG survey carried out in 2006, the direct economic losses were evaluated at USD 38.5 million yearly (USD 50 million in 2020)106. With a similar methodology, based on prevalence and mortality data due to NWS myiasis recorded in the 2014 DGSG survey, annual losses of USD 42 million were estimated (USD 47 million in 2020), material gathered from an unpublished draft by Grupo de Trabajo Ejecutivo (INIA, SUL, MGAP) (private collection; unreferenced). Furthermore, the benefit of the NWS fly eradication for Uruguayan livestock farmers, based on a model from Texas A&M University, was estimated at USD 99 million in 2000 (USD 154 million in 2020 adjusted for inflation)128. This model included costs reduction due to eradication (i.e., insecticides, drugs and veterinary care, inspection and surveillance, labor, animal mortality, among others), as well as increases in production (i.e., animal weight, meat, milk, among others). Considering the entire economy, annual losses could increase up to between USD 278 million and USD 1,233 million in 2020 dollars per year (USD 210 million in 2005, according to Wyss estimates cited in FAO/IAEA 2018, and USD 794 million in 2000, based on the Texas A&M University model).
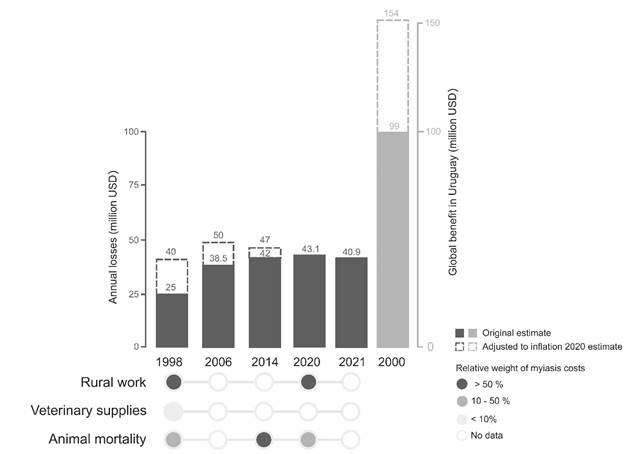
Figure 1: Annual losses, discriminated by category, due to myiasis caused by the New World Screwworm fly (NWS), Cochliomyia hominivorax. The gray bar (year 2000) shows the expected global benefit of the NWS eradication in Uruguay
In the most recent CBA carried out in Uruguay, Köbrich Grüebler129 and Baraldo130 estimated losses in the same range as the previous DGSG surveys. The first one estimated an annual cost of the NWS myiasis of USD 52.2 million, although the calculation includes cattle and sheep of a border area, up to 25 km from the frontiers with Argentina and Brazil, which would also benefit from an eradication program in Uruguay. Adjusting for this border area, the myiasis cost for Uruguayan livestock farmers was estimated at USD 43.1 million99. A detailed static simulation model on disease prevention, surveillance and treatment was developed to estimate the impacts of the NWS fly and to evaluate the benefits of an eradication program. It is important to note that, although the parameters are mostly based on assumptions and estimates from qualified informants, it appears robust according to expert criteria. Like in the previous studies, the main cost identified was rural working time spent on surveillance and diagnosis of myiasis activities. However, only 12.5% of the working day (1 hour/person/day) was imputed to NWS surveillance, according to experts. The study considered that surveillance time varies with farm size, for which farms were classified into small (less than 50 ha of surface), medium (50 to less than 200 ha) and large (200 or more ha). According to this, 1.72 million of working days (USD 22.3 millions) are dedicated to activities of infested animals’ treatment, search and separation of animals with wounds that could lead to NWS myiasis, which is equivalent to 0.096 working days per head of cattle. This estimate is lower, and therefore more conservative, than that by Hernández and Piaggio131, who calculated 0.158 days/year for surveillance per head of sheep or cattle. Also, the time in minutes for each preventive and curative treatment activity was considered as parameter in the model, in addition to the cost of veterinary supplies (USD 12.7 millions). Additionally, animal deaths and loss of leather value were considered (USD 17,2 million). Based on Köbrich’s work, Baraldo estimated the annual cost of the NWS myiasis in Uruguay at USD 40.9 million, and developed a CBA model for the eradication program proposed.
Indirect losses are also generated in Uruguay, as shown by 2006 and 2014 DGSG surveys: the adoption of inefficient herd management practices in order to avoid the highest incidence season of myiasis, as well as a loss of animal welfare. Additionally, NWS represents a risk to exports, because of food safety and live animal trade issues. As mentioned before, Uruguay was indicated as the source of the NWS fly introduced in Libya some decades ago132 and some commercial restrictions with this country remain impose.
4. Control strategies
Chemical insecticides are still the main strategy to control livestock ectoparasites worldwide, and the NWS fly is not an exception. Usually, disease management strategies based on chemical insecticides are farm-based relying on personal efforts and empirical knowledge about local resistance of the parasite under treatment to select the most suitable commercial product, which have different success rates. The indiscriminate usage of few compounds, mostly organophosphates and pyrethroids, has led to an increase of resistant individuals in natural populations, which in turn decreases these compounds efficacy, and thus making the control increasingly challenging133)(134)(135. Because of the reactive nature of this approach, as it is applied once the disease has been detected, this strategy will hardly reduce target pest populations. Alternatively, Area-Wide Integrated Pest Management (AW-IPM) is a systematic proactive approach that can integrate control strategies as the Sterile Insect Technique (SIT) or newly emerging biotechnological tools.
4.1. Insecticide usage
Mutations in target sites altering enzyme sensitivity to a chemical compound are the predominant molecular mechanisms of insecticide resistance, but also the metabolic detoxification pathways play a role in processing the insecticide. Independently or combined, these mechanisms confer resistance to all insecticide classes available136.
Organophosphate insecticides (OP), diethyl-OPs and dimethyl-OPs have been widely used to control infestations caused by NWS larvae. Non-synonym mutations (i.e., mutation that alters the amino acid sequence of a protein) in the NWS fly carboxylesterase E3 gene (also known as ChαE7) enable the hydrolysis of organophosphate substrates62)(137. It has been found that the substitution of a glycine for aspartate at position 137 (G137D) in the E3 enzyme of Diptera species confers broad spectrum resistance to OPs, mainly diethyl-OPs138. As well as the replacement of a tryptophan by leucine/serine at position 251 (W251L or W251S) has been associated with low resistance level to diethyl-OPs and high resistance level to dimethyl-OPs and may be related to cross-resistance to pyrethroid insecticides139. These mutations were detected in a wide region of Brazil and Uruguay, in a high occurrence in several locations65)(89, although whether resistant individuals due to the phenotypic expression of such mutations are circulating in this region has not been investigated.
Currently, in Uruguay, DILAVE-MGAP has approved 60 topical insecticides based on four kinds of active principles: phenylpyrazole (Fipronil), pyrethroid (Cypermethrin), organophosphate (Dichlorvos, Diazinon), and neonicotinoids (Imidacloprid) to control NWS larvae. These insecticides reached the MERCOSUR (N76/96) standard where a 100% efficacy must be proved, and the standard WAAVP140, where a 100% therapeutic efficacy and 90% persistent efficacy must be proved. Systemic insecticides of the macrocyclic lactones (Ivermectin and Doramectin) did not demonstrate therapeutic or prophylactic activity against NWS larvae, and are still under investigation.
A survey done in Artigas, Uruguay, revealed that most farmers (91%) use commercial insecticides, while a small fraction (9%) use both commercial and homemade insecticides (usually a mix of some kind of oil with a repellent). This questionnaire also pointed to a decrease in product effectiveness and 15% of the participants stopped using certain products. In general, farmers based on their empirical knowledge suspect that treatment with spray and powder fails (~55-60% curative fails) more than liquid (~70% curative success) and ointment (~70% curative success) insecticides141.
4.2. History of SIT-based control programs
The idea of reducing wild populations of a pest or parasite species by releasing sterile insects (i.e., the sterile insect technique, or SIT) and thus controlling them was independently conceived by Alexander S. Serebrovskii in Russia, Federic L. Vanderplank in Tanganyika (now Tanzania), and Edward F. Knipling in the USA during the 1930s and 1940s142. Knipling observed the extreme sexual aggressiveness of the NWS fly males, and that females refuse to mate more than once, realizing that if sexual sterility could be induced in males, and if a vast number of them could be sterilized and released in the field, then NWS natural populations would be eventually suppressed142)(143. The SIT is a genetic control approach based on the Mendelian inheritance of sterility produced by dominant lethal mutations generated by ionizing radiation144, that imposes birth control to further reduce the target pest populations145. Its application requires mass rearing of large numbers of the target insect under laboratory-controlled conditions, exposing them to ionizing radiation to induce sexual sterility, and releasing them successively into target wild populations on an area-wide basis. The NWS fly was the first insect parasite to be reared on an artificial diet enabling very large insect numbers for its study146. The SIT is usually integrated as a component of Area-Wide Integrated Pest Management (AW-IPM) programs145)(147 where the density of the target populations is initially reduced, eliminating already mated females with auxiliary methods such as insecticides148. Four kinds of AW-IPM integrating SIT can be deployed: suppression, eradication, containment and prevention149. A suppression program aims to maintain pest/parasite populations below an agreed and acceptable economic injury level and/or prevalence level. Eradication implies the elimination of a species from an area, usually an isolated local population. Nevertheless, the term eradication is restricted to the global extinction of a species. Containment is defined as the measures in and around an infested area to prevent spread of a pest/parasite, usually adopted to avoid the spread of alien species, or to consolidate progress made in an ongoing eradication program (e.g., current NWS fly program in Panama). The prevention strategy are the measures in and/or around a pest/parasite free area to avoid its introduction.
The NWS fly eradication from North and Central America is one of the most successful programs worldwide. Started in 1957 to rid the south-eastern USA and extended during the following ~40 years to eradicate it from the USA, Mexico, and Central America up to Panama24)(150)(151. By 1984 the goal of eradicating it to Mexico’s Isthmus of Tehuantepec was achieved152. At the request of livestock producers in southern Mexico and Central America, in 1986 the eradication campaign was extended to the Yucatán Peninsula and bordering countries153. Eradication was declared by steps as follows: Mexico 1991, Belize and Guatemala 1994, El Salvador and Honduras 1996, Nicaragua and Costa Rica 1999, and finally Panama 2006, where since 2004 a permanent barrier is maintained in the Darien region -along the Panama-Colombian border- to avoid reinfestation of endemic NWS from South America122)(151)(154)(155)(156. During these campaigns it has been shown that winter temperatures and rainfalls have a great negative impact on the appearance of NWS outbreaks and could be of great help to succeed in the eradication50)(157. Also, the typical low population density of C. hominivorax in the tropics was a key factor for the eradication157.
Although the APHIS (The Animal and Plant Health Inspection Service, USA) official recognition was in 2006, it was in June 2021 that the OIE (World Organization for Animal Health) published Panama’s January 2020 self-declaration of NWS-causing myiasis freedom. In Panama, the Panama-United States Commission for the Eradication and Prevention of Screwworm, or COPEG (www.copeg.org), constructed a smaller insect mass rearing facility in the early 2000s at Pacora, and the larger facility at Tuxtla Gutiérrez was closed in 2012142. The Panama facility was inaugurated in July 2006 and after three years of installation of equipment, biological safety measures, and staff training in rearing and insect sterilization the biosecurity facility mass production of flies began in March 2009. Currently, COPEG is the only existing NWS fly production facility in operation. With a maximum production capacity of 100 million sterile insects per week, it is currently producing and releasing 20 million sterile flies per week to provide biosecurity for the Plant and maintain the Permanent Biological Prevention Barrier.
An event that deserves to be highlighted was the above-mentioned accidental introduction of the NWS fly during the late 80’s in Libya, from where the screwworm was eradicated using SIT in 1992158. Although the NWS outbreak source remains unknown, it was initially suggested that it could have entered in a shipment of 236,000 live sheep from Uruguay in 1988. Uruguay as the NWS source of the Libya outbreak was discarded since the shipment arrived after the NWS cases had been already reported132. Despite this, the government of Libya banned the importation of live animals from Latin America, and until this date it was never re-established with Uruguay, despite the country being actively trading with other Arabian countries.
Currently, there is no official control program for the NWS fly in Uruguay or other country in South America. The strategies to reduce myiasis’ destructive effects rely on farm owner’s decisions, both chemical treatment and animal management (i.e., dehorning, castration, caravanning, calving, branding). Also, on zoos and parks responsible authorities, as well as veterinarians and medical doctors from private clinics and hospitals. The implementation of an AW- IPM based on SIT for the NWS fly in Uruguay has been largely discussed and is under evaluation. Some actions have been taken in order to evaluate the SIT usage in this region, and during 2007-2009 the governments of Brazil, Paraguay and Uruguay, supported by IDB (International Development Bank) and COMEXA (Mexico-American Commission for the Eradication of the Screwworm), carried out a demonstration on the Brazil-Uruguay border. This pilot program was conceived and undertaken to establish the basis for future programmes in the MERCOSUR countries127)(159)(160, and encompassed two phases: Phase 1) preparation, human resource training, and society communication; Phase 2) pilot dispersion of sterile flies. The main purpose was not the parasite eradication, but technology transfer and SIT field test on the region.
The first action of this pilot program was a mating compatibility study among the factory J06-strain, originally from Jamaica, and wild populations from Uruguay. Then, the field work was executed between January 23 and May 15, 2009, spanning 17 weeks, in an area covering 100x60km (100x30km in each country) with centroid in the cities of Artigas (30°24’24.54” S, 56°28’39.34” O, Uruguay) and Quaraí (30°23’33.13” S, 56°27’13.40” O, Brazil). Pre-dispersion happened the first two weeks, dispersion during the next 13 weeks, and post-dispersion the last two weeks. Sterile flies were sent from Tuxtla Gutierrez, State of Chiapas, Mexico, by COMEXA, and quality controls were done when they arrived at Artigas Airport in order to evaluate that delivery did not affect the sterile pupae.
For NWS fly monitoring, egg masses were collected in wounded animals, in addition to 10 gummed paper traps with Swormlure-4 (i.e., lure used in traps that release a strong odor to attract screwworm flies, a chemical mix including dimethyl disulfide, benzoic acid, indole, and phenol) as bait were distributed throughout the area and sentinel sheep were placed in 10 farms (five in each country). Differences in egg mass quantity between both countries were interpreted as due to a higher NWS fly population in Uruguay, which would occur as a function of the higher livestock density, mainly sheep, that doubled the Brazilian sheep stock in the region.
The sterility per week, measured as the proportion of sterile/fertile egg masses in sentinel animals, reached in the pilot area was directly related to the native NWS fly population and its oviposition rate, ranging from 1.53% in the second week of dispersion to 25.45% in week 11. When analysing the data within each country separately, the maximum sterility was in Brazil, with 40.7% in week 11. The demonstrative test was considered a success127 and the outcomes were promising, especially when considering that sterile flies were released during summer in the highest density peak of wild flies.
After this pilot project to demonstrate the SIT effectiveness in the Uruguay-Brazilian border, sterile flies were used to investigate natural barriers to NWS fly between Argentina and Uruguay in 20187. In collaboration with SENASA (Servicio Nacional de Salud y Calidad Agroalimentaria), Argentina, and the advice from USDA-ARS and USDA-APHIS scientists, and assistance from COPEG technicians, the role of the Uruguay River as a potential barrier for NWS fly crossing was evaluated. NWS sterile pupae shipped from COPEG, Panama, were marked with distinctive colors and released on both sides of the river in the region of Fray Bentos (releasing point: 32°53’23.57” S, 57°59’33.83” W, Uruguay) and Puerto Unzué (release point: 32°55’36.41” S, 58°14’23.60” W, Argentina). No marked flies crossing the Uruguay River were captured in three traps disposed in each country, at 4.3 km, 12.9 km and 15 km away from the release point in Uruguay, and at 6.2 km, 5.2 km and 13.9 km away from the release point in Argentina. The authors hypothesized that rivers can serve as barriers and riparian habitats along rivers can serve as corridors for the movement and dispersal of flies7. But it is necessary to take into account that the recapture percentages of stained flies were low, as previously found in Panama (156): 0.08% (8/10,000) of 1st, 0.05% (5/10,000) of 2nd and 0.07% (7/10,000) of 3rd dispersions in Uruguay, and 0.07% (7/10,000) of 1st, 0.03% (3/10,000) of 2nd and 0.02% (2/10,000) of 3rd dispersion in Argentina.
4.3. Biotechnology applied to NWS control
4.3.1. Transgenesis
There is an increasing need for improving the efficiency of the NWS fly eradication and prevention programs. The most common strategy based on insecticides tends to be inefficient to control insect populations at low density161 or where their application is made difficult by the landscape, favoring the reemergence of populations. In contrast, SIT is more efficient in low-density populations because they explore sites inaccessible to insecticides. But the current SIT releases both sterile males and females, even though male-only releases may be 3-5 times more effective at reducing local populations than bisexual sterile releases, since sterile males do not get distracted by county-released sterile females162.
Development of male-only strains for SIT programs was approached by transgenesis in two separate studies. The first consisted of a conditional female lethal transgenic strain of NWS based on the overexpression of the tetracycline-repressible transactivator (tTA) in females163, causing lethality, possibly due to ‘transcriptional squelching’ or interference with the ubiquitin-dependent proteolysis. Binding of tTA to the tetracycline operator (tetO) was strongly inhibited by the addition of tetracycline to the diet, providing a switch-off system. Only females expressed a functional tTA protein. Some of the homozygous strains produced exhibited suitable mass rearing and fitness characteristics. However, females were removed at the third instar larvae/pupae stage after they had consumed the larval diet. Additional saving in production costs might be obtained with a sexing strain that removes females from the mass rearing before they start feeding. Therefore, the next step was to develop a strain where females die early in development164 in a two-component genetic system: a) a Driver, containing an early promoter of an embryonic cellularization gene regulating tTA expression; and b) an Effector, containing a tTA-regulated pro-apoptotic gene. Transgenic strains were created carrying one of the two constructs and then homozygous lines for Driver and Effector are crossed to each other. Homozygous strains reared in a diet lacking tetracycline will develop males while those reared in a diet containing tetracycline will develop both sexes. All strains produced only males on a restrictive tetracycline feeding regimen. The females died at embryo or first instar larval stages. Evaluation of fitness characteristics, important for mass rearing, showed that one of the two-component strains and the all-in-one strain were particularly promising candidates to use in NWS control programs.
Despite the successful development of these female lethal systems, the strains generated yet need to be field tested before releasing in the wild. Also, there is a potential for development of resistance to lethal transgenes, for example, some commonly occurring genetic variation in fruit fly, Drosophila melanogaster, has been shown to provide almost complete protection from lethality induced by the tTA over expression system165.
4.3.2. CRISPR/Cas-based technology
The advance of genetic engineering brings the possibility of more complex genetic approaches for insect pest control. In this regard, the incorporation of CRISPR/Cas9-based systems95 could significantly improve the existing toolkit of NWS molecular methods. Briefly, Cas9 is an endonuclease that produces a targeted double-strand break in a DNA sequence guided by a single-stranded RNA complementary to the DNA target sequence (Figure 2a). The generated double strand break can be repaired by two types of mechanisms: the non-homologous end joining pathway (NHEJ), in which case the process commonly results in the introduction of deletions and/or insertions (collectively called indels) at the break site. Or, if supplied with a repair template, with a sequence complementarity to either side of the damaged region, homologous recombination (HDR) can occur to repair the break incorporating the template (Figure 2b).
Paulo and others96 established the first protocol to generate site-specific modifications in the NWS fly genome using the CRISPR/Cas9 system, targeting and disrupting the transformer gene (Chtra), producing intersexual female flies that show different levels of masculinization in their genitalia, while male adults show normal phenotype. As an important sex determination gene, required for normal female development, Chtra could be an interesting potential target for genetic control systems. In addition, the recently published whole-genome assembly of the NWS fly61 could allow the identification of other potential target genes that could be used in future genetic control programs based on CRISPR/Cas9.
The CRISPR/Cas9-based gene-editing system can be implemented in NWS management control for improving transgenic sexing strains designed for use in potential SIT programs. The use of this new technology avoids the concerns regarding the transgene being inserted in a random unpredictable place of the genome.
Additionally, the CRISPR/Cas9 technology can be used to design a gene drive system. Gene drives are naturally occurring selfish genetic elements that can increase the odds that they will be inherited (Figure 2c). Many researchers have suggested that these elements might serve as the basis for ‘synthetic gene drives’ capable of spreading engineered traits through wild populations166. Austin Burt167 was the first to propose gene drives based on site-specific ‘homing’ endonuclease. To build a CRISPR/Cas9 based gene drive, both Cas9 and the RNA guide must be inserted as a genetic construct in place of a sequence it can cut. If it can cut this sequence in organisms with one modified and one natural locus, reliably inducing the cell to copy the construct, and avoid being too costly to the organism, it will spread through susceptible wide populations. The drive can spread this genetic construct or disrupt existing genes166.
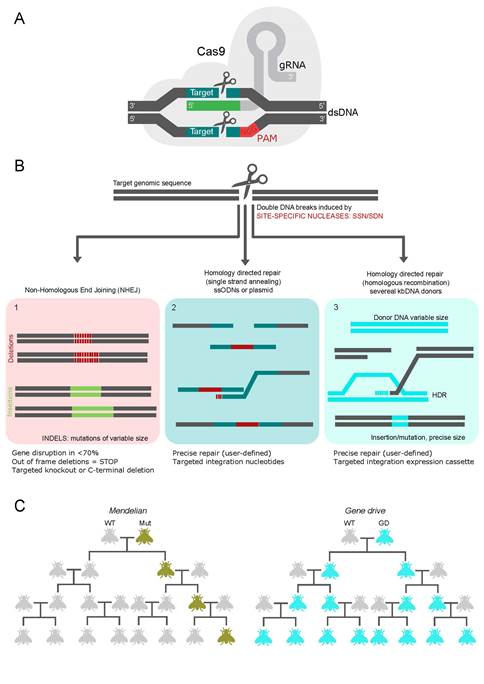
Figure 2: Main outcomes of genome-editing by CRISPR/Cas9. A) Target-specific single guide RNA (sgRNA, in green) form a complex with the endonuclease CRISPR-associated protein 9 (Cas9, in gray), and upon recognition of a DNA sequence (complementary to the sgRNA), just before a 5’-end recognition PAM sequence (NGG, where “N” is any nucleotide), the Cas9 promotes double-strand breaks (DSB) in double-stranded DNA (dsDNA) 3-4 nt 5’ of the PAM. B) DSBs repair pathways triggered by gene-specific nuclease: In 1, a mutation is generated, a gene is inactivated. Quasi-random mutation at the target site, indistinguishable from the natural mutation, no DNA fragments are left in the final product. In 2, a targeted nucleotide change edits a gene using a small DNA template sequence. In 3, a DNA fragment is inserted. The transferred fragment can be recognized, and additional DNA fragments remain in the final products. C) Genealogies comparing the spread of genetic changes or mutations as expected by the Mendelian (leftmost) and gene drive (rightmost) inheritance mechanisms
For NWS population control, one promising strategy is to implement a population suppression gene drive that could reduce the size of the target population by disrupting genes causing infertility or lethality only when both copies are lost168)(169. Whether a standard gene drive will spread through a target population depends on molecular factors such as homing efficiency, fitness cost, and evolutionary stability, but it is also sensitive to specific ecological variables such as mating dynamics, generation time, and other characteristics of the population170.
Because any consequences of releasing gene drives into the environment would be shared by the local if not global community, research involving gene drives capable of spreading through wild-type populations should occur only after a careful and fully transparent review process. This should include independent scientific assessments of probable impacts and fully inclusive public discussions171. Possible ecological effects can be assessed by performing contained field trials with organisms that have been engineered to contain the desired change but do not possess a functional drive to spread it. Finally, the prevalence of the gene drive in the environment could in principle be monitored by targeted amplification or metagenomic sequencing of environmental samples.
5. Environmental impact of control programs
Environmental protection aims to avoid and/or repair ecosystem damage by focusing on relevant adverse effects on biotic or abiotic resources, which has an impact on conservation and would affect ecosystem components or its sustainable use. In this context, it is also important to consider the magnitude of the adverse effects caused by the NWS fly, and the regulatory decisions should take into account the ecological consequences of the application/non-application of control measures when trying to mitigate the damage caused by this pest insect.
Invasive and parasitic species can cause significant damage to the environment, agriculture and human health, and there are often few tools available to control their populations172. The eradication or population suppression of the NWS fly, as well as the control program per se, could have an impact on the ecological landscape, and whether the control actions are acceptable depends on the balance between environmental and health damage caused by this ectoparasite versus unintended off-target effects172. The characteristics of selected control strategy and its management determine the information required to identify and evaluate the effects on the environment. In SIT-based eradication, the analysis focuses on possible environmental negative effects due the NWS fly elimination and specific activities of the control program. For biotechnology-based techniques (e.g., transgenics and CRISPR) it is necessary to evaluate the possible environmental negative effects of the genetic modification itself, in addition to the likely impacts of the species eradication. Decision making is supported by risk analysis, which includes a comparative problem formulation approach to define the risk hypothesis that will determine whether the control plan being evaluated may harm people or the environment172. Based on environmental protection goals, characteristics of the control plan that may cause adverse effects are identified. For each possible negative impact, its probability of occurrence and possible consequences are estimated based on literature review; NWS fly biology and ecology, and results of NWS eradication programs carried out in other countries. The objective of the risk analysis is to manage the tension between a desire for caution regarding the risk of intervention and the worry about the risks of non-intervention173.
5.1. Environmental impact of NWS fly eradication
In order to assess the possible impacts of NWS fly eradication, the Ministry of Agriculture of Uruguay (MGAP) through a consultancy is evaluating the potential, both negative and positive, environmental and social impacts of the NWS fly eradication. A preliminary analysis indicated that the NWS fly eradication in Uruguay would not have significant negative impacts and these would not exceed social and environmental benefits. Likewise, to reduce uncertainties, it was confirmed the usefulness of gathering information at the national level of the NWS fly population size, distribution and dynamics, through the fly monitoring, as well as generating national information on the distribution and population dynamics of species through monitoring for possible ecological impacts at agricultural landscapes and natural fields, before, during and after the execution of the control program. This kind of information will make it possible to evaluate different negative and positive potential socio-environmental impacts.
Potential impacts associated with ecological interactions include the likely population growth of NWS fly wildlife hosts, as well as the reduction of stress caused by myiasis in domestic and wild hosts if this ectoparasite is eradicated. The potential effect of the NWS fly as a regulator of wildlife can be evaluated as positive in the case that native warm-blooded animals are beneficiated, but negative when alien species considered pests are the beneficiaries. Special attention called the effect on feral swine114, and a preliminary analysis estimates a low risk since its population density depends on other more determining factors than the NWS fly. The animal stress reduction propitiated by the elimination of myiasis must be considered as positive, independently if the species is native or alien, under the current animal welfare standards.
The interaction network with other species could also be affected once the NWS fly is removed and the ecological dynamics of its predators, pathogens, commensals and/or mutualists can be negatively influenced as well as competitors can be positively influenced. No significant risk is expected for known NWS fly predators (e.g., ant, beetles, spider) given the low probability of exclusive dependence on it as a food source, since they are generalist species174)(175. Similar to what must happen with the NWS fly commensal, mutualist and pathogenic species. For competing species, there are no records at the national level of species that could occupy the NWS niche, so the risk could be characterized as non-existent. The absence of tight specific ecological interactions, leading to a dependency relationship with the NWS fly, is supported by its seasonal population dynamics and the geographical localization of our country in the species distribution border, that may not represent an optimal area. Despite this, the absence of ecological interactions with specialist species hypothesis should be evaluated with data collected during a species monitoring planned within the eradication program under discussion by the authorities.
Another biotic environmental factor that must also be contemplated is its role as pollinator in the long-time176. However, interactions with flowering plants in the role of adults as exclusive pollinators have not been identified. In summary, a preliminary risk analysis of the environmental impact of an NWS fly eradication program in Uruguay indicates that it would not result in significant alterations on ecosystems and ecological interactions. The NWS fly eradication programs have been implemented in wide areas (from USA to Panama) since the 1950s with no significant negative environmental impacts reported on the specialized literature. Although, as far as we know, no specific monitoring studies have been conducted, 70 years without reports of negative ecological impacts have contributed as an indirect proof with the implementation of control programs.
5.2. Environmental impact of the control program for the NWS fly eradication
Control strategies may involve certain activities that require to be analysed in order to determine whether they could have a negative impact on the environment. In the case of SIT-based eradication, insecticides usage during the first phase of the program and dispersion of many sterile flies were considered. Regarding the use of insecticides, that could cause potential contamination of soils and waters, on side with the mortality of non-target animals (i.e., pollinators), the potential impact is limited to the first phase of the program, because of the increasing use of topical and/or systemic insecticides for prophylaxis and myiasis treatment, as the needed way to reduce wild populations density before sterile flies’ dispersion. In a long-term, the increased usage of insecticides during this first phase will not be higher than what has been currently applied, and once Uruguay is declared free of the NWS fly its usage will decrease drastically, being necessary only to control some sporadic myiasis and suspicious cases.
During dispersal and maintenance phases the effect on air traffic due to the dispersion of millions of sterile flies could negatively affect other species, such as avifauna, because of the disturbances caused by aircrafts and the novel traffic pathways. However, its impact could be considered non-significant since the traffic bulk would be also concentrated in the first phases of the program.
Recently, genetically modified or preferentially inherited (GD, for gene drive) organisms have been proposed as a new tool that could be used to control or eradicate parasite or vector species. Gene drive systems (see subheading 5.3.2 and Figure 2c) allow the introduction of genetic elements at a higher frequency than expected, which can rapidly change genotypes in target populations with consequences on species fitness. For example, the control of the dengue vector Aedes aegypti by releasing genetically modified sterile males, described in Araújo and others161, in Grand Cayman (2009 and 2010), Malaysia in 2010 and Brazil in 2011, with an efficiency greater than 80% population suppression, can be approached by a GD system.
The environmental risk assessment of interventions based on novel biotechnological tools, like transgenesis and gene editing, requires having genetic and technical information about the specific strategy used to generate the organism as well as information about target and non-target species. Ecological and evolutionary data would be required such as hybridization or horizontal gene transfer between target and non-target species, the evaluation of potential target sites in the genome of non-target species, food web structure, behavioral and demographic data177, as well as the mating system and gene flow between populations (e.g., dispersal ability and anthropogenic dispersal)178.
Finally, yet important, the geography of Uruguay nestled in the Pampas biome, extending from southern Brazil, through the center of Argentina until the south of the Province of Buenos Aires, where no dry boundaries exist, represents a challenge to determine the releasing locations and merits an informed community consent. Release into the environment could include transboundary movement and replacement of off-target populations, with potential human/animal health impacts. Therefore, eventual regional coordination should be considered.
6. Regulatory framework for intervention on natural systems
The control or eradication of the NWS fly, despite its favorable cost-benefits relation for the livestock industry, economy and finally the whole society, should be based on actions taken within the appropriate legal framework.
6.1. Current regulations
Genetic modified organisms (GMOs) are regulated in most countries and covered by international agreements such as the Cartagena Protocol under the United Nations CBD. However, excessive cautious and restrictive GMO regulations that often prevent the use of new technologies could prolong the risk of animal suffering, loss of food security, environmental imbalance using insecticides and unspecific household products. Research and development are advancing faster than regulation and CRISPR opens doors in many fields of production and environmental conservation (described below). The technology stands out for reducing time, improving the use of resources, and reducing costs to generate new varieties, involving private companies and institutions. Vegetal GMOs have been planted on millions of hectares and commercialized for a long time, but regulation is variable worldwide, with very different endpoints. Based on past experiences of innovation, it is desirable that GMO applicants and regulators interact to achieve the benefits of innovation while cautiously avoiding unacceptable risks.
In Uruguay, the existence of enacted legislation (Decree No. 353/008) that regulates the importation and various GMO uses -including research uses- within our territory- provides a safe, necessary and reliable regulatory environment for the application of biotechnological innovations. This legislation, in addition to providing a suitable environment for the application of safe biotechnologies, enables collaborations between countries in the long term. The enacted legislation also represents evidence of public deliberation on the use of genetically modified organisms within the country, and an important cultural and logistical asset that enables the use of innovative, state-of-the-art solutions179)(180. A well-designed risk assessment helps to manage the tension between the desire for caution regarding the risk of intervention and concern about the risks of non-intervention172.
6.2. Regulatory aspects of genome editing
Genome editing by gene-specific nucleases such as CRISPR/Cas is a versatile tool that generates variations in the recipient genome at specific target sites. The CRISPR/Cas editing system is composed of two clearly differentiated elements, the nucleotide fraction usually constituted by the single guide RNA (sgRNA), and the enzymatic fraction represented by the Cas9 endonuclease (see subheading 5.3.2 and Figure 2). Once introduced into the cell the specifically designed sgRNA guides the Cas9 protein into the specific sequence in the genome to be modified by making a double cut in the target DNA. From this point on, the non-homologous end joining (NHEJ) or the direct homology repair (HDR) pathway of cellular repair systems introduce the desired mutation (Figure 2b). The NHEJ pathway promotes insertion or deletion mutations (indels) that generate, by changing the correct reading frame, a termination codon that disrupts the gene. If the edition aims to modify the gene sequence without truncating it, the cell must be provided with a repair template carrying the intended mutation and the HDR pathway is followed. The repair of DNA cleavage by gene-specific nucleases results in variants at the target site, and three types of alterations can be distinguished. In the absence of donor of DNA the NHEJ repair introduces base pair changes or small insertions/deletions resulting in frame-shift mutations that cause premature stop codons and mRNA degradation. The exact change cannot be predetermined and is almost random at the target site. When a DNA donor is present, either single stranded oligonucleotides (ssODNs) or double stranded DNA, the DNA homology repair by single-stand annealing or homology recombination occurs, respectively181. In the cases that large DNA elements of foreign origin are introduced it is usually considered transgenic182. It is not the case for CRISPR-based genome editing when foreign DNA is not added (e.g. knock out models), and then it is generally accepted that should not be required additional regulatory oversight than other breeding varieties. The introduction of large repair templates (e.g. knock in models) requires further regulatory oversight and specific approval. The regulatory system for genome editing differs between countries, and while some are seen as more innovative, others are more restrictive or conservative.
6.3. NWS fly editing
The development of gene-edited strains of the NWS fly by CRISPR/Cas to be used in a control program could be considered beneficial as it is species specific, and mitigates damage to the environment, economy or health of wildlife, domesticated and human life, although it is ‘invasive’ to some extent. Since GD applications aim to release organisms that become established in the environment and may spread throughout different habitats, countries have the responsibility to assess transboundary risks and liability for damage caused by such releases. It is likely that bilateral and regional measures will first establish approaches before harmonization at higher international levels172.
7. Economic impacts of a control program
Economic impacts of disease control programs are usually approached by the integration of economic and epidemiological information. As before, cost-benefit analysis (CBA) is one of the most widely applied methods in this field, at the individual producer level and at the whole livestock sector level allowing detailed estimates of diseases costs for farmers as well as of controlling them, comparing the income changes in different scenarios. But it could be important to consider economy-wide impacts, for example if prices -including exchange rate- can be affected by the disease (especially when it implies changes on the access to international markets). Therefore, tools that also capture spread indirect effects (e.g., through changes in prices and/or input-output relationships between productive sectors) help to gain broader insights on disease’s economic impact183. It has been suggested the integration of epidemiological information and models with Input Output (IO), Partial Equilibrium (PE) models, multi-market models, or Computable General Equilibrium Models (CGEM)184. CGEM are mathematical representations of the entire economy that enable the estimate of direct, indirect and induced impacts of external factors, changes in technology or in policies, on both macroeconomic and sectoral indicators. It allows analyses of changes in the levels of production, exports and imports by sector, and additionally captures the reallocation of resources between productive sectors, including labor employment, in response to changes in relative returns.
The NWS fly eradication programs in North and Central America required the economic justification for its execution and several appraisals based on consumers and producers’ surplus theory have been developed and operationalized through CBA10)(121)(122)(123)(124)(185)(186)(187)(188. All analysis supports the economic profit of the NWS eradication, with high net returns for farmers and the whole economy in those countries. According to Wyss122, in addition to the annual benefits obtained by the American, Mexican and Central American farmers thanks to the eradication, there is a multiplier effect (by a 3.5 factor) of the livestock sector towards other productive sectors. This annual impact on the whole economy was estimated at USD 3,000 million for the United States, USD 1,100 million for Mexico and USD 297.8 million for the Central American countries (USD 4,660, USD 1,708 and USD 463 million in 2020, adjusted for inflation). Also, it was concluded that the benefit for consumers was similar to that of producers, so that the sum of both components results in a very important general effect on the economy. Furthermore, in Libya the economic evaluation showed that the eradication program was a remarkably profitable investment, with a cost-benefit ratio of 5 in the infested zone, and 10 for the whole of the economy125)(132)(189.
In South America pre-feasibility studies of three eradication proposals for the Mercosur region concluded they were highly convenient from the socio-economic point of view, with internal rates of return (IRR) between 121% and 157%, and cost-benefit ratios of between 2.97 and 3.91190. The sensitivity analysis showed that even if program costs or investments increased 50%, or benefits fell 50%, the Net Present Value (NPV) continues to be positive. One of the main expected impacts is the economic efficiency improvement through a drastic reduction in costs of labor and veterinary supplies for the treatment of affected animals.
As mentioned before, up to date, there is no official program for the NWS fly control in Uruguay; however, livestock farmers and authorities have agreed on its importance. Its economic feasibility, financing alternatives, environmental and social impacts and other institutional concerns, such as governance, are under active discussion and analyzes are taking place. Two alternative SIT-based programs were evaluated, one proposed by FAO/IAEA, that represent a first step in of a Subregional Strategic Plan for the NWS eradication in South America99)(191)(192, and another by USDA/COPEG/ MGAP and the national agricultural institutions.
The FAO/IAEA strategy consists of a progressive eradication from south to north of Uruguay and gradually advancing towards the north of the continent. It is based on the establishment of four regions (East-West strips) in the Uruguayan territory and later containment barriers in the borders with Argentina and Brazil to keep the country free of NWS, similarly to what has been done in the Panama-Colombian border. This program aims to eradicate the NWS fly in 7 years, releasing a maximum of 55 million sterile NWS pupae per week, with a simple cumulative cost of USD 154.6 million in the first 10 years, that has been taken as evaluation horizon. A CBA of the FAO/IAEA program for Uruguay estimated direct losses caused by the NWS fly on livestock over a 14-year time horizon, concluding that in Uruguay it would achieve an economic net present value (NPV at 7.5%) of USD 97.924 million (ranging from USD 86.2 to USD 158.4 million) and an economic IRR of 27% (the minimum IRR of 14.7% exceeds the social discounted rate of 7.5%)129. The discounted benefits always exceed the discounted costs with a cost-benefit ratio of 1.87 (ranging from 1.71 to 2.26) and a payback period of 8 years, indicating the convenience of implementing the proposed eradication program. Additionally, through a CGEM calibrated for Uruguay OPYPA-MGAP simulated the macroeconomic and sectoral effects of the FAO/IAEA program193. The working hours fall for surveillance and treatment of NWS fly and avoided livestock deaths were modeled as a productivity increase, and the lower expenditure on veterinary supplies for myiasis treatment and prevention was modeled as a reduced use of chemical products coefficient. The costs and investments required to implement the eradication program were included in the model as an increase in government consumption and investments, and the import of sterile pupae was introduced through a transfer from the Uruguayan government to the rest of the world (amounts were taken from)129. The impacts of three alternative financing scenario were also considered. All simulations showed that a successful eradication strategy would have positive impacts on the whole economic activity, particularly on livestock farming and associated productive sectors. Furthermore, in the labor market, the average salary and employment of the entire economy would receive positive impacts as well. Total exports, as well as the general government collection, would increase due to the effects of direct, indirect and induced effects of the eradication program.
The USDA/COPEG/MGAP program aims to achieve the NWS fly eradication within three years, one year of preparation and two years of sterile fly release. Uruguay will be divided into four regions (East-West strips) and the sterile fly releasing will begin from the south. This proposal plans to use between 8 and 26 million pupae/week, significantly less than that estimated for the FAO/IAEA program. Because the eradication has been projected to be by region, it is expected that benefits are partially and progressively achieved. Prevention and treatment (and its costs) will stop as autochthonous cases of myiasis disappear, but vigilance for reintroductions must be maintained for an indeterminate period. Sterile flies and field inspectors would be permanently required to maintain the myiasis free status, at least until Argentina and south Brazil do not reach the eradication. It was estimated a total program cost of USD 40 million and about USD 4.5 million per year for the border barriers, whereas in an evaluation horizon of 10 years the cumulative cost of the program would be USD 62.5 million130. According to the CBA, the project would achieve a NPV at 7.5% (i.e., ~USD 146 million) and an economic IRR of 96% with a cost-benefit ratio of 4.3, indicating that is a highly profitable investment. Program risk was assessed by sensitivity analysis of several negative scenarios. First, a longer program, which implies higher operational costs as well as a delay in the eradication benefits. Even in the worst scenario where eradication takes 9 years the NPV is still positive. Second, a higher number of sterile flies to be released, considering that FAO/IAEA proposal, established twice the number of flies per linear nautical mile (6,000 vs 3,000). Although these flies’ requirement is three times the estimated, NPV is still positive. Third, as already mentioned, saving labor is the main benefit of an NWS fly eradication program, so overestimating the working days to surveillance and disease treatment could over-evaluate the NPV of the program. A sensitivity exercise was carried out re-estimating project indicators when the benefit from labor savings fell to half of that estimated by experts; in all scenarios the NPV is positive. Fourth, an important part of the potential benefits results from avoiding deaths, saving veterinary supplies and surveillance time in sheep herds. However, the national sheep stock showed a downward trend along the last 20 years, and in 2019 it was reduced to half the herd of 2000194)(195. If this trend continues in the next few years, part of the benefits of the NWS eradication program would be reduced. An extreme scenario in which the sheep herd falls 10% cumulative annually (~3 times faster than observed from 2000 to 2019) was carried out, and the NPV is still positive. In summary, these univariate analyses showed that none of these factors alone would compromise the economic feasibility of the program.
To further stress the project feasibility a joint sensitivity analysis was conducted taking factors two by two and in none of the scenarios the NPV turned zero or negative. However, when three or more factors take unfavorable values together, scenarios arise in which the discounted program costs equal or exceed the benefits, turning the program economically inconvenient. Despite the economic feasibility of the NWS fly eradication program in Uruguay has been supported by these analyses (see Köbrich Grüebler129, Baraldo and Durán130 and Ackermann and others193), the occurrence of unfavorable scenarios, with negative values of several factors simultaneously, must be considered in the decision-making process.
8. Perspectives and knowledge gaps
The ultimate goal of reducing the incidence of myiasis in Uruguay -which has been extensively discussed in the present review as a promising action to increase animal and human health by controlling or ideally eradicating the NWS fly- can be achieved by two complementary strategies. On the one side, the well-known SIT-based strategy is being actively discussed by authorities, experts and livestock producers, and some efforts have been made in order to its implementation in the near future. On the other side, a CRISPR/Cas-based strategy to generate a gene drive system aiming to introduce sterility in the NWS fly wild populations is under research. Both strategies can be used in a complementary way in the same eradication program, and/or the CRISPR/Cas-based strategy can be the substitute of the SIT-based program when it finalizes, designed specifically to keep the sanitary status achieved. An advantage of the CRISPR/Cas-based is its national development, in collaboration with US institutions, that warrant the complete control of the strategy. Once NWS fly gene drive has been produced and tested, local institutions, authorities and ultimately livestock producers can be the owners of this biotechnology. Another advantage of the CRISPR/Cas approach is the know-how that will be acquired by local scientists and scientific institutions, opening the possibility to locally develop new strategies for other ectoparasites and/or vectors important for animal and public health.
In the case of the NWS fly, although it is also a human health issue, its importance for meat producing countries, particularly Uruguay and region, has been well explained in the present review. Highlighting animal welfare aspects -especially considering the animal suffering caused by myiasis-, the implementation of eradication measures is clearly relevant. Insecticide usage has proved to be inefficient for the NWS fly containment at a broad geographic scale and its diverse natural habitats. In contrast, environment-friendly strategies such as SIT and/or gene drives are more efficient for NWS fly control in low-density populations and have been specifically outlined and could prove effective in limiting its spread. The gene drive strategy is considered the least environmental impacting technology, while developing logistical and scientific capabilities. Innovation in insect and disease control methods reveal a constant concern for human and animal health, plants, and ecosystems. On the other hand, the country must adapt its biosafety regulatory framework for animal and microorganism regulation, developing specific methodologies for problem formulation in risk analysis.
According to Oliva and others179, the success of control strategies that integrate sterile insect releases as part of broader pest/parasite control or health management programs depends largely on gaining public understanding and acceptance. Integration of SIT must be done carefully and requires proper long-term planning, as potential areas for the implementation of control programs are diverse and culturally distinct well-tailored forms of information, dissemination, and public interaction strategies are required. The public concern about GMOs will also influence the debate on different SIT and/or biotechnological based strategies, or on non-intervention in the environment. On this point, Esvelt196, with an open and responsive approach to science, mentions that for the public to have a voice in decisions that will affect the shared environment, research on preferential inheritance and gene drive should be conducted openly from the earliest stages. This should be specifically conducted by a community of trusted scientists, considering the concerns that may arise from the social community and who, informed about the topic, can guide future research and decision making.
According to Collins180, it is not straightforward to reconcile the argument for not intervening at all in nature with GD proposals used to alleviate the burden of parasitic or infectious diseases in animals and humans, conserve species, or increase agricultural productivity. Ultimately, reconciling these competing interests and values will determine how intrusive one is willing to be in shaping populations and ecosystems. It also highlights the importance of using multidisciplinary and interdisciplinary approaches to decision making related to the development and application of preferential inheritance technology. The US National Academy of Sciences, Engineering, and Medicine committee180 called for a responsible scientific approach that requires ongoing evaluation, assessment, and education regarding social (defining types of stakeholder involvement), environmental, regulatory, and ethical considerations.
From the social sciences point of view179, the release of genetically modified insects involves three essential conditions: a genuine willingness to involve the public (which might not be evident to scientists and institutional partners), conducting research publicly, and allocating sufficient human and financial resources for public participation activities.















