1. Introduction
Monilinia fructicola is a fungal pathogen responsible for fruit brown rot, blossom and twig blight in many species of Prunus1. This disease is considered as a major limiting factor in stone fruit production worldwide1)(2)(3. Susceptibility of freestone peach (Prunus persica (L.) Batsch) to infection by this pathogen has been shown to change during fruit growth4. Thus, peach and nectarine (Prunus persica var. nectarina Maxim.) fruits are prone to M. fructicola infection during the early stage of rapid expansion of the pericarp (stage I), and then again at harvest maturity (stage III). Developmentally intermediate stage II fruit (pit hardening) is less susceptible to infection1)(3)(5)(6.
Fruit of Brazilian clingstone peach Bolinha possesses a high level of brown rot resistance due to differences in the architecture of epidermal tissue and a thicker cuticle with high phenolic content2. The accumulation of phenolic compounds is one of the typical defense responses of plants against the pathogens attack7. Chlorogenic and caffeic acids are major phenolic acids in the epidermis and subepidermal cell layers of peach fruit2.
Currently, the main strategy to control diseases in fruit plants is through pre-harvest fungicide use8)(9. However, synthetic fungicides offer risks to human health and to the environment. In addition, certain fungicides are no longer efficient due to the emergence of resistant strains of Monilinia spp.10)(11. Thus, there is a growing need to develop alternative approaches for disease control9)(12, like the use of abiotic elicitors to induce the fruit resistance8)(9, phosphites among them13. Phosphites are not-phytotoxic compounds that have a high fungicidal activity14)(15. Their mode of action may be direct or indirect, through the activation of plant defense mechanisms by phytoalexin production14)(16)(17)(18. Changes in the susceptibility of peach -Monilinia fructicola- pathosystem could be associated with chemical inhibitors, including phenolic compounds, and the preventive treatments with phosphites could activate plant defense mechanisms.
The aim of this work was to evaluate the effect of potassium and calcium phosphites on the content of total phenols, chlorogenic acid and changes of fruit susceptibility to Monilinia fructicola.
2. Materials and methods
2.1 Plant material and fungal inoculum
Peach (Prunus persica L. Batsch) fruits, Flordaking and Elegant Lady, from the eea inta San Pedro (33°44'12.7"S, 59°47'58.2"W) experimental station, were used to evaluate the effect of potassium and calcium phosphites (ffk and ffca, respectively) in three developmental stages: I) green fruit (gf), II) pit hardening (ph), and III) harvest time (ht).
A fungal inoculum of Monilinia fructicola provided by the Laboratory of Phytopathology from eea inta San Pedro (Buenos Aires, Argentina) was cultivated on Potato Glucose Agar medium, and incubated at 25 °C, during 15 days until spore formation. Then, a suspension of 1×10 6 conidia mL-1 was prepared for fruit inoculation.
2.2 Experimental treatments
Phosphites were applied in the field, biweekly, from a month after full flowering till harvest, in both cultivars. Three treatments were assayed: a) 0.3%-ffk (300 mL per hL, cs 12.9% assimilable phosphorus; 16.3% soluble potassium), b) 0.3%-ffca (300 mL per hL, cs P2O5 29.7%, Ca 12.7%), and c) control (water), in a block design with 3 replications. The day after applying the treatments, 20 fruits per treatment of each replication were harvested and taken to the laboratory. The developmental stage of the fruits was determined based on their size by using a caliber, and on the evaluation of the pit and embryo formation, according to the ease of opening it with a knife. Ten of the harvested fruits per treatment of each replication were inoculated on the suture with 30 µL of a conidial suspension of M. fructicola isolate grown on Potato Glucose Agar; while the remaining 10 fruits were not inoculated. The conidial concentration was measured with a hemocytometer, and the suspension was adjusted to the desired concentration (1×106 conidia mL-1) with sterile water. Inoculated and non-inoculated fruits were kept for 6 hours at room temperature, until they were taken to the laboratory to extract phenols.
2.3 Phenolic compounds determination
After treatment, samples were transferred to the Molecular Biology laboratory from Faculty of Agricultural Sciences of the National University of Rosario (Santa Fe, Argentina), where the extraction and determination of phenols were carried out based on the methodology described by Coseteng and Lee19.
Extracts were obtained from the skin of fruits, inoculated with M. fructicola under controlled laboratory conditions and without inoculation, for both cultivars.
A 50-g sample of fruit skins was homogenized in a blender with 100 mL of 80% ethanol and boiled for 5 minutes under a hood. The extract was filtered through paper and the residue was mixed with an additional 100 mL of the same ethanol solution and boiled for 10 minutes to re-extract phenolics. The extracts were combined, and the solution was allowed to cool before it was made up to a final volume of 250 mL.
The alcohol extract was diluted to obtain an absorbance reading within the range of the standards (10-100 mg mL-1). One mL of the diluted extract was added to 10-mL distilled water. Two mL of Folin-Ciocalteau phenol reagent was added, then the sample was mixed, and after 5 minutes, 2-mL saturated Na2CO3 solution was added and shaken. Absorbance at A640 nm was measured in a uv-Vis Spectrophotometer (Perkin Elmer, Ohio, usa) after 1 hour. The amount of total phenolics was calculated from a standard curve of catechol prepared at the same time.
Chlorogenic acid was determined by a modification of the procedure described by Mapson and others20. It was necessary to dilute the alcohol extract to obtain an absorbance reading <1.00. Two mL of 5% sodium molybdate solution in 50% ethanol was added to 10 mL of the diluted alcohol extract. After mixing, a 10 mL aliquot of the above solution was mixed with 2 mL of 50% ethanol, and the absorbance at 370 nm was read in a uv-Vis Spectrophotometer (Perkin Elmer, Ohio, usa). The concentration of chlorogenic acid was determined from a standard curve (10-50 µg chlorogenic acid mL-1) prepared at the same time. The results were expressed as µg chlorogenic acid per g fresh fruit.
2.4 Data Analysis
Experiments were carried out in triplicate. The data were subjected to analysis of variance, and the significance of the differences between means was estimated by the Tukey´s test (p≤0.01 or p≤0.05).
The entire data set that supports the results of this study is published in the article itself.
3. Results and discussion
The two cultivars studied showed different behavior regarding the amount of total phenols determined. The Flordaking showed differences (p≤0.05) between treatments for the content of total phenols expressed as µg per g fresh fruit. General means were: ffca = 374.8 ± 47.04 a, ffk = 362 ± 16.85 a, and Control = 306.55 ± 58.65 b. This effect was observed in gf and ph stages, but not in ht. On the other hand, the treatments did not express differences in the content of chlorogenic acid. The total phenol content did not vary between inoculated and non-inoculated fruits, although chlorogenic acid did, which showed a fall in the presence of the pathogen (Figure 1). This pattern was observed in the different stages of development for all treatments. The accumulation of phenolic compounds is one of the typical defense responses of plants against the attack by pathogens7. This behavior could be due to the possibility that the pathogen developed attack pathways that counteract the defense of the fruit.
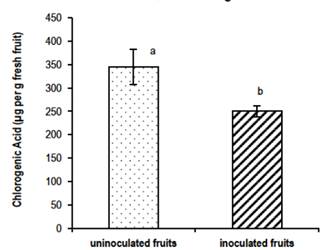
Figure 1: Content (µg per g fresh fruit) of chlorogenic acid in the skin of inoculated and non-inoculated fruits, Flordaking
The contents of total phenols and chlorogenic acid were not affected by the phosphite treatments in Elegant Lady. Total phenols in the skin of inoculated fruits (413 µg per g fresh fruit) decreased more than half compared to non-inoculated fruits (867 µg per g fresh fruit) in ph and ht status (p≤0.01). In gf stage, the content of chlorogenic acid was higher in the absence of the pathogen (p≤0.01); however, this effect was reversed (p≤0.01) in the ht stage, unlike Flordaking (Figure 2). There are known reports in which changes in phenolic metabolism have been found after plant-pathogen interaction7.
Both metabolites showed significant seasonal behavior (p≤0.01) for the two cultivars studied, with a peak in ph and a drop at ht (Figure 3), matching the time of lower and higher susceptibility of the fruit to the disease, respectively. Lee and Bostock1 found higher levels of total phenols in the skin of fruits in stage II of growth and significantly lower in harvest maturity (end of stage III). In addition, they identified chlorogenic acid as the main phenolic compound in the skin of peaches, which declined during the transition from stage II to stage III of the fruit, in agreement with the results presented in this work.
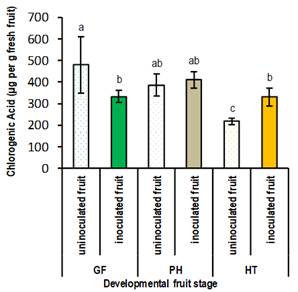
Figure 2: Content (µg per g fresh fruit) of chlorogenic acid in the skin of inoculated and non-inoculated fruits, in different stages of developments for the cultivar Elegant Lady: green fruit (gf), pit hardening (ph) and harvest time (ht)
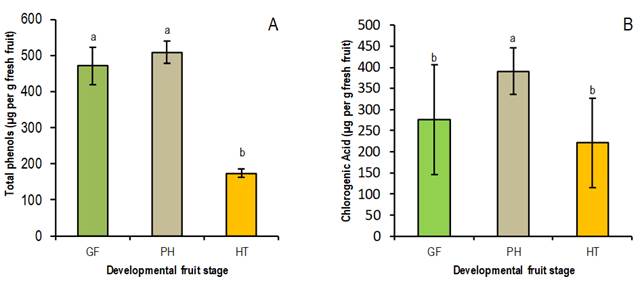
Figure 3: Evolution in the content of total phenols (A) and chlorogenic acid (B) (µg per g fresh fruit) in the fruit skin of cultivar Flordaking during its development stages: green fruit (gf), pit hardening (ph) and harvest time (ht)
Phosphites can stimulate rapid production of phytoalexins, which are important mechanisms of plant defense14)(21)(22. In this work, the preventive application of phosphites did not show a clear relationship regarding the content of secondary metabolites.
4. Conclusions
The content of total phenols and chlorogenic acid presents a seasonal behavior, which accompanies the curve of peach fruit susceptibility to infection by Monilinia fructicola. However, up to now, it is not possible to confirm that this differential susceptibility is exclusively associated with the content of these chemical inhibitors.
Phosphites showed random responses in both metabolites and cultivars. We did not observe activation of defense mechanisms against preventive applications. Further investigations are necessary to define the effect of phosphites at the enzymatic level in the peach-Monilinia fructicola pathosystem.