1. Introduction
Trichogramma wasps (Hymenoptera: Trichogrammatidae) are known as primary egg parasitoids of a wide range of hosts, especially Lepidoptera. Most of the other known hosts belong to endopterygote orders (Diptera, Coleoptera, Hymenoptera, Neuroptera). In general, most Trichogramma vary in length from 0.4 to 0.6 mm; females tend to be slightly larger than males1. Studies on Trichogramma began in the early twentieth century, when Flanders2 discovered the possibility of rearing it in a fictional host, Sitotroga cerealella (Olivier) (Lepidoptera, Gelechiidae)3. Their success is favored by their very short generation times, simple mass rearing systems, persistent economic efficiency and technologies adapted for commercial use4)(5)(6. For years, the use of Trichogramma has been an essential part of pest management strategies in protecting crops against economically important agricultural and forest pests. Currently, they are the most widely produced and released natural enemies throughout the world5)(7)(8. A key attribute of Trichogramma, as other egg parasitoids, is the fact that they not only attack the host eggs, but also kill the host in the egg stage and consequently kill the pest before it can damage the crop4.
Trichogramma wasps are applied using inundative biological control methods, which consist of releasing hundreds of thousands of native or exotic biological control agents per hectare to obtain direct pest control; however, agents are not expected to persist from one cropping cycle to the next9)(10. Releases of Trichogramma are performed manually or mechanically. Manual releasing requires considerable labor, therefore, for extensive areas where labor is expensive, ground or aerial mechanical releases are employed. For this, release containers of different materials are used to protect Trichogramma from predators and weather conditions. Nonetheless, they can be released without protection7.
There are more than 200 known species of Trichogramma present in the six biogeographic regions of the world (Palearctic, Oriental, Nearctic, Neotropical, Afrotropical and Australian)11. Trichogramma identification, using the male genital tract as diagnostic character12)(13, allows potential species discrimination. The use of reproductive compatibility studies14)(15, allozyme variation16)(17 and molecular methods18 as tools for identification has continued to refine Trichogramma species taxonomy.
In Uruguay, six species of Trichogramma have been identified from collections of sugar cane, corn, cotton, rice, vine, apple, pear and olive tree19)(20. Among the reported species, Uruguay has T. pretiosum Riley as the most widely distributed in the country, collected in the first five crops mentioned above, and parasitizing, according to the crop, Diatraea saccharalis Fabricius, Heliothis zea (Boddie), Alabama argillacea (Hübner) and Argyrotaenia sphaleropa (Meyrick)19)(21)(22. When released in crops, it was found also parasitizing Rachiplusia nu (Guenée) and Anticarsia gemmatalis Hübner and, in laboratory studies, Helicoverpa armigera Hübner and Tuta absoluta (Meyrick) (unpublished information). T. pretiosum is present in almost all biogeographic regions in the world. In the Americas, it is one of the most commonly collected, especially in agricultural and other disturbed habitats. It has 240 host records1.
Trichogramma pretiosum has been subject of studies at the Entomology Unit of the Faculty of Agronomy of Uruguay (Udelar), regarding its biology23)(24, taxonomy22, morphology25 and parasitic effectiveness in grapevine21, lotus seedlings26 and tomato. In recent years, biological and utilization studies of T. pretiosum have been carried out in soybean crops (2003-2015: transgenic soybean, 2016-2020: non-transgenic soybean). Interest in soy was based on economic, social, environmental and health impact of this crop in Uruguay, which went from 9,000 hectares in the 1999-2000 growing season27, to a peak of 1,334,000 hectares in 2014-201528, and then declined to 966,000 hectares in 2018-201929. This area occupied by soybean cultivation continues to be very important in Uruguay (39% of the total area allocated to crops in 201929), and at an economic level it was the country's third export item in that year30.
Soybeans are attacked by various lepidopteran pests, such as R. nu, A. gemmatalis, Crocidosema aporema (Walsingham) and Helicoverpa armigera (Hübner). The first two species are generally the most important for their defoliating action. They present a different behavior, thus while A. gemmatalis is located in the upper layer of the conepia, R. nu prefers the lower layer. Furthermore, the type of damage is different between the two species; when feeding, A. gemmatalis does not respect the secondary veins, while R. nu eats between the secondary veins and gives a “net” appearance to the damaged leaflet31)(32)(33. To prevent these damages, the application of chemical insecticides is commonly used. As in other parts of the world, such sanitary management has negative effects, such as the rapid selection of pest strains that are resistant to a given active ingredient34, or the reduction of natural enemies, especially when using non-selective insecticides35)(36. Furthermore, in large areas, this practice has been related to the invasion of non-native arthropod species37. This has justified promoting biological control methods.
The selection of T. pretiosum was based on the fact that, in the laboratory, it presented the highest fertility parasitizing eggs of Ephestia kuehniella Zeller (alternative host used for these wasps multiplication) and of A. gemmatalis deposited on soybean plants, when compared to T. exiguum Pinto and Platner and T. galloi Zucchi, species also present in Uruguay (study carried out by P. Maignet, J. Seguret and C. Basso, unpublished). Starting in 2003, on-field trials were carried out on small soybean plots within the framework of an agreement between the Faculty of Agronomy (Udelar) and Barraca J.W. Erro S.A., a company dedicated to the commercialization and export of soybean. For these studies, samples of T. pretiosum were multiplied and conditioned for their release in paper envelopes in the Entomology Unit of the Faculty of Agronomy. In order to increase scientific and technological capacity, as of 2010, Bioline Agrosciences (ex Biotop), a French company that produces and commercializes natural enemies of crop pests, joined this venture. Within the framework of this alliance, samples of the selected line of T. pretiosum were sent to France, for their massive multiplication and later re-entry to Uruguay to carry out the releases. For the release of the parasitoids in the culture, 20 mm diameter cellulose capsules were used, containing eggs of E. kuehniella parasitized with Trichogramma. These capsules have small openings for the exit of adult wasps. For capsule distribution in the cultivation area, traditional machinery used in the application of soybean chemical insecticides (commonly known as ‘mosquitos’) was employed, to which a machine supplied by Bioline Agrosciences was attached for its distribution in crops. The releases and technical evaluation were performed by the company Entoagari. In 2018, the company Biophilia registered the biological insecticide Tricholine Maxi in the Ministry of Agriculture, Livestock and Fisheries, containing 1,000 eggs of E. kuehniella parasitized by T. pretiosum per release capsule (selected line from Uruguayan sample). Of the total parasitoid number, half are found in a more advanced stage of development within the eggs of E. kuehniella, which ensures two moments of emergence of adult wasps from each capsule when they are deposited in the crops, approximately separated between 7 and 15 days, depending on the environmental temperature.
In this study, the pest control obtained through releases of T. pretiosum was compared with the conventional use of chemical insecticides, evaluating different doses of Trichogramma and number of released capsules per hectare, different number of releases in the culture cycle and ground or aerial releases (with or without release capsules, respectively). This would allow defining a strategy for the use of T. pretiosum in soybean cultivation, according to the needs of control effectiveness and economy of force.
Potential predation of E. kuehniella eggs parasitized by T. pretiosum when dispersed in the crop without capsule protection was taken into consideration and the phenomenon was studied in the same parasitoid-releasing cultures, as has been verified in the laboratory with species of ants collected in soybean crops in Uruguay38.
2. Material and methods
2.1 Rearing and preparation of the Trichogramma
The colony of T. pretiosum used in this study was originally established from a thelytokous female emerged from a parasitized egg of Helicoverpa zea (Boddie) (Lepidoptera, Noctuidae) collected in the style of a female corn inflorescence. The collection took place in February 2014 in a crop located in Colonia department (Uruguay). The morphological identification of the species, using characters from the male genitalia12)(13, was carried out by Bernard Pintureau, before converting some females into arrhenotokous to obtain males using antibiotics(1 ).
The Trichogramma were reared in E. kuehniella eggs at 25 ± 1 ºC and 70-80% HR in the Entomology Unit of the Faculty of Agronomy, Udelar (Montevideo, Uruguay). In 2015, a sample from that colony was sent to Bioline Agrosciences (Livron-sur-Drôme and Valbonne, France), where it continued to multiply under the same conditions.
To meet the trial needs, the multiplied Trichogramma were sent to Uruguay in a diapause state, where they were stored at 3 ºC in the Jumecal cooperative facilities (Montevideo). Before the releases, the mentioned state was broken by a temperature increase.
2.2 Study areas, experimental design and treatments
The studies were carried out in three soy producing establishments located in an area demarcated by the cities of Dolores, Palmitas and Ombues de Lavalle in the department of Soriano: San Patricio (33°41'29.0"S, 58°04'58.9"W), Villa Trigo (33°31'19.1"S, 58°01'36.0"W) and Estancia Chica (33°46'46.7" , 57°44'52.2"W). In all cases, an undetermined soybean variety belonging to group 6 of maturity was used.
A plot trial including six treatments was implemented in each of the sites. The treatments varied in the number of releases, number of Trichogramma per capsule, number of capsules per hectare, dose of Trichogramma per hectare and mode of release. One of the treatments consisted in the application of chemical insecticides, with active ingredients and an opportunity criteria typical of the technical advisers of the cultures involved, which acted as a standard control39 (of known effect), providing a reference of comparison with treatments based on T. pretiosum (Table 1).
Table 1: Description of treatments according to number of releases, number of Trichogramma per capsule, number of capsules per hectare, dose of Trichogramma per hectare, and type of application
| Treatments | N. Releases | N. Trich : cap | N. cap : hect | Dose Trich : hect | Application type |
| 1 | 2 | 1,000 | 100 | 200,000 | Ground |
| 2 | 3 | 1,000 | 100 | 300,000 | Ground |
| 3 | 2 | 2,000 | 50 | 200,000 | Ground |
| 4 | 4 | ---- | --- | 200,000 | Aerial |
| 5 | 2 | 1,250 | 50 | 125,000 | Ground |
| 6 | Use of chemical insecticides | Ground | |||
Trich = Trichogramma, cap = capsule, hect = hectare
Ground applications were made using a capsule dispensing machine model manufactured by Bioline Agrosciences. A DJI Inspire 2 model drone was used for the aerial releases. A dispenser (model manufactured by Bioline Agrosciences) of eggs of E. kuehniella parasitized by wasps Trichogramma was attached to the drone. In ground application, capsules containing Trichogramma were used at two stages of development. Eggs of E. kuehniella with Trichogramma were dispersed using the drone in a single moment of development. The plots in ground applications covered 20 hectares of cultivation and in the aerial applications 50 hectares of cultivation.
Ground release dates of Trichogramma were (in all three sites): first release January 17-18, 2020, second release February 4-5, 2020, third release (treatment 2 only) February 17-18, 2020. The dates of the drone releases were: first release January 16-17, 2020, second release January 24-25, 2020, third release January 30-31, 2020, and fourth release February 10-11, 2020.
2.3 Estimation of the abundance of lepidopteran larvae and percentage of plant defoliation
In the experimental plots, six weekly samplings of lepidopteran larvae were taken using the cloth method on 1 m of furrow, with 10 repetitions per plot and date. In the counts, the larvae were differentiated by species and grouped into small and large according to whether they were in larval stages 1-3 or 4-5, respectively. The natural enemies collected in the same samplings were also counted, recording the presence of spiders, Orius sp, Nabis sp, lacewings and coccinellids. In addition, the defoliation percentage caused by the larvae on five plants was estimated in each meter of sampled furrow, according to the scale proposed by Iannone40, which defined six levels of eaten foliar area: 5, 10, 15, 20, 25 and 30%. The percentage of lepidoptera eggs parasitized by Trichogramma was not estimated, given the difficulty of collecting postures massively in the foliage of soybean crops. The evaluation period was from 21 January to 28 February 2020, and included soybean plants that developed from phenological status R1 to R5.
2.4 E. kuehniella egg predation parasitized by T. pretiosum
E. kuehniella egg predation parasitized by T. pretiosum was measured when deposited in soybean crops without being protected in release capsules. For this, in the same selected soybean crops in each experimental site, 40 groups of 20 eggs each were deposited in yellow cardboard of 10 mm long by 5 mm wide, half of them stapled in soybean leaves and the other half placed next to the ground, maintained by a small wooden piece (a toothpick), on two ocassions (16 to 18 January, and 11 to 13 February 2020). In addition, in the same sites as in the previous trial, 20 pieces of cardboard of the same size, with an egg of E. kuehniella parasitized by T. pretiosum in each, were stapled to soybean leaves.
Half of the pieces of cardboard were removed within 24 hours, and the remaining 48 hours after deposit, and the level of predation (without the presence of egg chorion remains that could indicate the Trichogramma emergence) was determined in the laboratory under stereoscopic microscope (Olympus model SZ51). The groups of eggs were classified into five levels according to the predation suffered: 0, 25, 50, 75 and 100%. The isolated eggs were distinguished depending on whether or not they had suffered predation.
2.5 Statistical analysis
The goodness of fit of the Poisson distribution was tested for the variables that include counts using the Kolmogorov-Smirnov test at a 95% confidence level. The differences in accumulated larval counts over time according to treatment were analyzed by site, adjusting a Generalized Linear Model41 with logarithmic link function. The variation in the number of larvae over time according to treatment was analyzed according to the monitoring date, including the three sites in the analysis, using the same methodology. The variance components were estimated using the maximum likelihood method. The means of each treatment were separated by DGC42 multiple comparison tests at a confidence level of 95%.
The defoliation percentage was analyzed by a non-parametric analysis of variance using the Kruskal-Wallis test with a confidence level of 95%. The mean ranges were compared according to the classification criteria: treatment and site. Each treatment was compared according to monitoring date. The treatments were compared considering the sites as repetitions. The comparison between sites was carried out including the defoliation percentage of all the treatments.
A cluster analysis was performed using the hierarchical method. The grouping was carried out through the average linkage method, using the Euclidean as a measure of distance. The analysis included all the collected variables and monitoring dates, using the treatments as classification criteria.
The statistical analyzes were performed with the InFostat 2018 software with R interface, and Origin pro-lab 2019b.
3. Results and discussion
3.1 Effectiveness of T. pretiosum release and of the application of chemical insecticides
The total number of lepidopteran larvae (A. gemmatalis plus R. nu) accumulated throughout the samplings of the three study sites was different (p<0.0001) when the sites and treatments were compared between each other. The interaction between site and treatment was also significant (p<0.0001). The lowest number of larvae was reached with treatment 6 (chemical insecticides), followed by treatments 1 (two ground applications, each of 100,000 parasitoids per hectare, 100 capsules per hectare), 2 (three ground applications of 100,000 parasitoids per hectare each, 100 capsules per hectare) and 4 (four aerial applications, each consisting of 50,000 parasitoids per hectare), that did not differ from each other (p < 0.05). Finally, the treatments with the highest number of larvae were treatments 3 (two ground applications of 100,000 parasitoids per hectare each, 50 capsules per hectare) and 5 (two ground applications of 62,500 parasitoids per hectare each, 50 capsules per hectare), that did not differ from each other (DGC test, p < 0.05) (Figure 1).
When performing the analysis by site, in all cases treatment 6 was the most effective, while treatments 3 and 5 had the highest number of larvae, although they did not differ from treatment 2 in Estancia Chica, from treatment 4 in San Patricio, and treatments 1 and 2 in Villa Trigo (DGC test, p < 0.05). In the global analysis of all data, the highest number of larvae per unit of the cloth method was 4.0 ± 0.37 in treatment 3 at the sixth sampling.
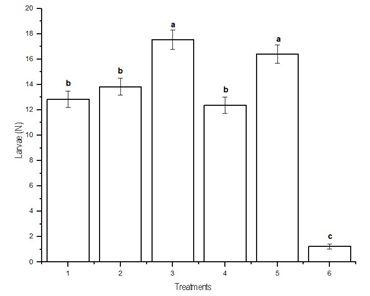
Figure 1: Average accumulated number by treatment of larvae of Anticarsia gemmatalis and Rachiplusia nu of all samplings (mean ± SD). Means fitted using a Generalized Linear Model (~ Poisson). Different letters indicate significant differences (DGC test, p < 0.05)
In plots under conventional management (treatment 6), one or two applications of chemical insecticides were carried out depending on the site, which implied using two to four different active ingredients of insecticides to control lepidopteran pests (Villa Trigo: Chlorpyrifos and Triflumuron, San Patricio: Bifentrin and Chlorantraniliprole, Estancia Chica: Triflumuron, Chlorpyrifos, Thiamethoxan-Bifentin and Chlorantraniliprole).
3.2 Effect of treatments according to Lepidoptera species and larvae size
The lowest average number of larvae of A. gemmatalis per sampling, analyzing the three sites together, was achieved with treatment 6, that was different from treatments 1 and 4, which did not differ from each other, and followed by treatments 2, 3 and 5, which did not differ from each other, and presented the highest values (DGC test, p < 0.05). On the other hand, the same analysis for R. nu discriminated the treatments into three groups: treatment 6 with the least number of larvae; treatments 1, 2 and 4 that did not differ from each other; and treatments 3 and 5, that did not differ from each other and presented the highest values (DGC test, p < 0.05). The collections of R. nu more than doubled A. gemmatalis in all the different samplings (Figure 2).
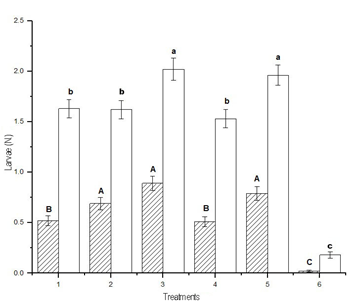
Figure 2: Average accumulated number by treatment of larvae of Anticarsia gemmatalis and Rachiplusia nu of all samplings (mean ± SD). Means fitted using a Generalized Linear Model (~ Poisson). Different letters indicate significant differences (DGC test, p < 0.05). Lowercase: comparison of means of R. nu. Uppercase: comparison of means of A. gemmatalis
When the analysis was performed according to the size of the larvae, the smallest number of small larvae was collected in treatment 6, which was different from treatments 1 and 4, which did not differ from each other, and followed by treatments 2, 3 and 5, which did not differ from each other and presented the highest values (DGC test, p < 0.05). The lowest number of large larvae per sampling was achieved in treatment 6, different from treatments 1, 2 and 4, which did not differ from each other, and followed by treatments 3 and 5, which did not differ from each other, and presented the highest values (DGC test, p < 0.05) (Figure 3). The highest average number of small larvae collected in sampling by the cloth method was 5.9 in treatment 5, and that of large larvae was 3.9 in treatment 3.
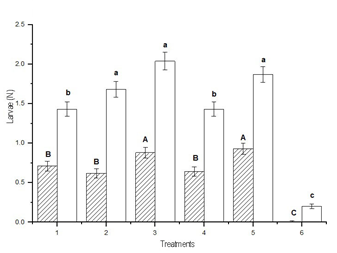
Figure 3: Average number per treatment according to size of the larvae of Rachiplusia nu and Anticarsia gemmatalis together (mean ± SD). Means fitted using a Generalized Linear Model (~ Poisson). Different letters indicate significant differences (DGC test, p < 0.05). Lowercase: comparison of means of small larvae. Uppercase: comparison of means of large larvae
3.3. Effect of the number of release points, number of releases and doses on the results with T. pretiosum
All the analyzes showed a lower control of treatment 3 compared to 1 (DGC test, p < 0.05), indicating a negative effect of the reduction from 100 to 50 in the number of release capsules per hectare, with the same dose and number of releases. This coincides with the indicated by Zachrisson and Parra43, who reported that the effective radius of action of T. pretiosum in soybeans extended 10 m from the point of release. On the other hand, Bueno44 found that this species dispersed 8 m in a soybean crop, a similar result if considering that small distance variations can be attributed to the characteristics of the parasitoid lines, the climate in the study region and the culture45. It has been shown that the dispersal distance of Trichogramma can be affected by the number of specimens released. Thus, for example, T. ostriniae Peng et Chang, which stands out for reaching the highest dispersion in the genus, is affected by the release dose46, while Fournier and Boivin47 found no release rate effects in T. pretiosum, which could explain its lower dispersal capacity compared to T. ostriniae.
When the dose was reduced (treatment 5), apart from the reduction of the release points (treatment 3), no difference in results was found between them (DGC test, p < 0.05). This would indicate that the dispersion capacity of the Trichogramma is the factor that negatively affects these two treatments compared to the rest.
A third release of T. pretiosum at a later stage of the culture cycle (treatment 2) was not reflected in better control of lepidopteran pest, when compared with two releases (treatment 1). This indicates that carrying out three releases would not be convenient, since it would increase the costs of biological control due to the higher dose of parasitoids and the costs of an additional application.
3.4 Defoliation percentage and abundance of larvae regarding the thresholds of chemical insecticides application
The lowest level of defoliation caused by Lepidopteran larvae, when the three experiment sites were analyzed together, occurred in the plots that received the application of chemical insecticides (treatment 6), and the highest occurred in the plots under treatments 3 and 5. The plots of treatments 1, 2 and 4 showed intermediate values (Kruskal Wallis, p < 0.05). The mean value of defoliation did not exceed 22.5% in all samplings (Table 2).
Table 2: Defoliation percentage according to treatments on four monitoring dates. Mean values (± SD) of the three sites (Villa Trigo, Estancia Chica, San Patricio)
| Treatments | Date 3. | Date 4. | Date 5. | Date 6. |
| 1 | 16.6 ± 5.9 b | 11.6 ± 2.4 c | 15.0 ± 4.1 c | 13.3 ± 2.4 c |
| 2 | 16.6 ± 5.9 b | 8.3 ± 2.4 c | 10.0 ± 0 b | 11.7 ± 2.4 b |
| 3 | 16.6 ± 5.9 b | 15.0 ± 7.2 c | 16.7 ± 6,3 b | 11.7 ± 2.4 c |
| 4 | 16.6 ± 5.9 b | 13.3 ± 2.4 c | 15.0 ± 4.1 c | 13.3 ± 4.8 c |
| 5 | 16.6 ± 5.9 b | 13.3 ± 2.4 c | 20 ± 0 d | 18.3 ± 2.4 c |
| 6 | 0 ± 0 a | 0 ± 0 a | 0 ± 0 a | 0 ± 0 a |
Dates 1 and 2: the defoliation percentage is zero. Different letters in the columns indicate significant difference by non-parametric analysis of variance (Kruskal-Wallis, p < 0.05)
Although the application of chemical insecticides allowed better results than the release of T. pretiosum, all cases were below the empirical threshold of 15 larvae per meter and 30% defoliation, commonly used in Uruguay to decide the application of chemical insecticides in soybean crops. The same conclusion is reached if considering the threshold of 20 large larvae and 30-35% defoliation up to the phenological state R2 and 15-20% from R3 to R6, determined by Perotti and Gamundi33 in Argentina, for soybean varieties of the same maturity group as the one used in this study. In assessing the damage to Lepidopteran larvae, producers and technical advisers should abandon the "zero damage" paradigm to foliage, which can only be achieved by repeatedly applying chemical insecticides, with the consequent risk to human health and environmental degradation. Studies by Perotti and Gamundi33 allowed to determine a decreasing trend in the yield of soybean crops with the increase in defoliation levels, above 27%, which is above the levels achieved with the use of T. pretiosum in all treatments. Given that this included the treatments where the number of release points per hectare was reduced, studies should be continued before ruling out these options, taking into account that the number of release points per surface influences the material and economic effort required to carry out biological control actions46)(48.
The positive results obtained with the release of this parasitoid are consistent with the indicated by Bueno and others49, that T. pretiosum is well adapted to a temperature range between 18 and 32 ºC and, therefore, it is potentially suitable for its use in biological control programs for A. gemmatalis and P. includens in different geographic areas that fit within that range. This would explain why T. pretiosum was the first egg parasitoid to be commercially available for lepidoptera control in soybean crops in Brazil50.
3.5 Aerial application and predation of E. kuehniella eggs parasitized by T. pretiosum
As previously indicated, the aerial application of Trichogramma wasps using a drone (treatment 4) achieved similar or better results than those obtained with terrestrial treatments with the same or higher doses and 100 release capsules per hectare (treatments 1 and 2). These results were obtained despite having verified a high predation effect of E. kuehniella eggs parasitized by T. pretiosum when they were expressly deposited in the crop.
The counts indicated that some groups of eggs had not been predated and others had been almost totally or completely predated, revealing that the results depended on the ability of the predator to locate the eggs. Based on this, results were analyzed in these two situations (with and without predation). Considering the data set, close to ground level, only 20% of the eggs survived 24 hours, and 6% remained 48 hours after being placed. At foliage level, 62% of the eggs remained 24 hours and decreased to 44% at 48 hours. On the other hand, 67% of the eggs placed individually in the foliage were not predated at 24 hours and 33% at 48 hours.
This egg predation, probably due to some ant species present in soybean crops in Uruguay, of proven voracity for that prey38, would force the release of Trichogramma that are close to emerging, in aerial applications, to reduce the exposure time.
To compensate for the fact that in each aerial application only one flight of Trichogramma emergence is achieved, while capsules achieve two, the need for more releases with drones than with “mosquitos” must be taken into account when comparing the logistics of both means of release. In the same way, it should be positively valued that the application with drones does not depend on the possibilities of immediate entry into the crop after episodes of rain, as happens with “mosquitos”. As a reference, in Brazil, where biological control in soybeans with T. pretiosum has shown great advance in recent years, the application with drone has been defined as one of the priorities of these programs51.
3.6 Natural enemies and the grouping of treatments
Considering the three sites, the accumulated number of natural enemies counted in the different treatments was not different (DGC test, p < 0.05). Differences between sites and interaction between site and treatment were verified. Only the number of spiders allowed discriminating the treatments in one of the sites (Estancia Chica), where the lowest values were reached in the plots under treatments 5 and 6, which did not differ from each other, while the remaining treatments had higher values without differentiating from each other (DGC test, p < 0.05).
The grouping of the treatments in clusters according to the Euclidean distance, when the total mean accumulated samplings of all the analyzed variables were considered (total larvae, total large larvae, total small larvae, total larvae of A. gemmatalis, total larvae of R. nu, spiders, Orius sp., Nabis sp., lacewings, coccinellids and defoliation percentage), allowed differentiating treatment 6 (use of chemical insecticides) from the rest of treatments. A cluster closer to this one, made up of treatments 4 and 2, and a more distant cluster made up of treatments 1, 3 and 5, where the latter two formed a sub-cluster, were determined. Globally, the treatments were ordered in a similar way as in the particular analyzes (Figure 4).
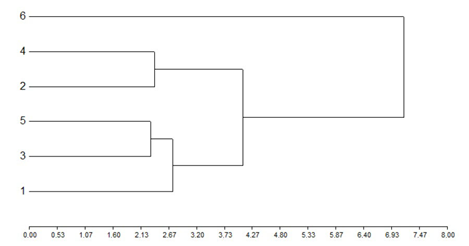
Figure 4: Grouping of the treatments in clusters according to the Euclidean distance, considering the accumulated mean values of the number of: larvae, large larvae, small larvae, larvae of A. gemmatalis, larvae of R. nu, spiders, Orius sp., Nabis sp., lacewings and coccinellids, and the defoliation percentage
4. Conclusions
The application of T. pretiosum under the inundative biological control method appears as a real alternative to chemical insecticides for the control of the main lepidopteran pests in soybean crops in Uruguay. With releases of this parasitoid, levels of larvae abundance or defoliation percentages with an impact on crop yield can be avoided according to bibliographic information. Two ground or four aerial releases appear as the best options for using T. pretiosum from the alternatives evaluated, even though predation of the parasitoids before their emergence must be taken into account in aerial applications.
The positive results of this study could stimulate initiatives for research of biological methods for the control of the stink bug Piezodorus guildinii (Westwood) (Hemiptera: Pentatomidae), which also demands repeated applications of chemical insecticides in soybean crops.
To enable the implementation of alternatives to the use of chemicals, the paradigm of “zero damage” in crops must be abandoned. Active public policies of incentives, training and awareness can help develop a disposition towards sustainable agriculture.
Consumers are increasingly demanding food safety and guarantees on environmentally careful production. Conventional control of crop pests is one of the sources of toxicity and contamination. Uruguay has the possibility of being among the countries that meet such requirements, and the application of biological control through T. pretiosum can contribute to differentiate and enhance our agricultural production.