1. Introduction
The E. dunnii (Maiden) is a diploid species (2n=22) that belongs to the Myrtaceae family1, originally from small populations in Queensland, Australia2, and grows well in temperate climates, showing good tolerance to frost. In Uruguay, eucalyptus plantations represent one of the most important economic activities, mainly due to cellulose pulp production and, to a lesser extent, solid wood production for furniture, and panels and chips manufacture. The most planted species are E. globulus, E. grandis and E. dunnii3. Genetic improvement is essential to obtain genotypes more adapted to our soil and climate conditions. Forest species have problems associated with the long period of juvenility until plants reach maturity and flowering; this is why a breeding program becomes slow, expensive, and complex4.
Considering the long reproduction cycle in these species, technologies have been developed to accelerate and shorten the breeding cycles; one of these technologies is polyploid induction. Polyploidy exists in nature and constitutes a way of generating new variability, mainly through the production of non-reduced gametes and interspecific hybridization5, with the potential for almost instant reproductive isolation, in addition to being a proven source of novel adaptation6)(7. Genome duplication is important for variability generation1 since the change in the number of gene copies results in changes in the morphology, structure, and physiology of new individuals8)(9. Different methods have been used to generate polyploid trees, the application of colchicine is among the most used. This compound has shown great effectiveness in the results.
Likewise, there are other compounds, such as trifluralin and oryzalin, which have the advantage of being used in lower concentrations and are also specific for plant cells10. Both oryzalin and colchicine work in a similar way, stopping the cell multiplication process. During mitosis, colchicine inhibits microtubule polymerization; this failure in the formation of microtubules stops mitosis before reaching metaphase, preventing chromosome segregation11, and determines that two sets of chromosomes remain in the same nucleus. To verify the effectiveness of the treatments, artificial induction of polyploids must be confirmed. Different approaches have been evaluated to identify duplicate individuals. In forest species such as poplar, the count of chloroplasts in stomatal guard cells was used as an indicator of the ploidy level12.
Padoan and others13 compared triploid and diploid Clementine mandarin stomata and found differences in stomatal cell frequency, size, and shape, as well as in the length of stomatal guard cells, and number of stomatal chloroplasts. They observed a positive correlation of these parameters with the ploidy level; these characteristics were used as morphological markers to identify polyploidy in many plant species. Xue and others14 compared the morphology, growth and development of Hanfu apple tree diploids and autotetraploids. They established differences between diploids and tetraploids in measurements obtained in photosynthetic parameters such as chlorophyll content and fluorescence, indicating better performance in tetraploid apple plants. While these measurements are useful markers for identifying polyploid individuals, the most widely used and widespread tool to confirm the ploidy level is flow cytometry (fc). Amano and others15 showed that it is possible to analyze a large number of samples in a short time with a high level of repetitiveness through this technique, being a fast and safe technique. It is based on the number of fluorescence being directly proportional to the amount of dna present in cells. To determine the content of nuclear dna, isolated nuclei are marked with fluorochromes such as propidium iodide, ethidium bromide or with 4′-6-diamino-2-phenylindole (dapi), prior removal of rna from the samples. The emission of fluorescence by fluorochromes can be quantified and the ploidy established with respect to control cell populations. About 90% of the tissue cells are in G0 and G1 phase of the cell cycle, i.e. their chromosomal load corresponds to the chromosome pairs of the species (2c). The other cells are doubling dna (G2) or have already duplicated it (4c) before mitosis16. The analytical ability, speed, and precision of this technique make it a very useful instrument for checking the ploidy level.
Another way to confirm the ploidy level in plants is chromosome counting, but this technique requires experience, specific knowledge and is time-consuming, so it can be applied to a limited number of individuals17. Artificial polyploids exist in some eucalyptus species: in a hybrid clone of E. grandis x E. urophylla, in E. globulus and E. grandis18. There are no reports of obtaining polyploid plants of E. dunnii.
The objective of this study was to adjust a polyploid induction methodology at E. dunnii, using in vitro culture.
2. Material and methods
2.1 Plant material
The plant material (clones and seeds) was supplied by the company Forestal Oriental S. A. Two types of explants were used: plant material introduced in vitro and seeds germinated in vitro; in both cases, the starting material was of the diploid E. dunnii species.
In vitro explants (ie): Four clones of E. dunnii, selected from different populations, were introduced in vitro from potted plants actively growing in a greenhouse on the Biotechnology Unit of INIA, Las Brujas. Apical and lateral buds were taken and superficially disinfected with 10% sodium hypochlorite with two drops of Tween 20, for 10 minutes. They were then rinsed three times with sterile distilled water. They were introduced into a culture medium containing the macroelements of Murashige and Skoog19 (ms), modified in the following components: 8.1 mM ammonium nitrate, 1.03 mM potassium phosphate monobasic, 1.41 mM magnesium sulfate, 4.4 mM potassium nitrate, 2.9 mM magnesium chloride. In addition, 3.4 mM calcium nitrate and 0.86 mM calcium chloride were included, the microelements corresponded to the woody plant medium wpm20) and vitamins21. The growth regulators used were: ba (benzyladenine) 0.4 µM, and naa (naphthalene acetic acid) 0.05 µM. The pH was adjusted to 5.8 before sterilization by autoclaving and 1% (w/v) sucrose was included per liter of culture medium.
Microshoots were subcultured every 15 days until reaching at least 200 explants of each diploid genotype; 20 to 25 explants were used per treatment. In total four clones were introduced.
Seed Explant (se): The seeds were sterilized in a 20% sodium hypochlorite solution with two drops of Tween 20 for 15 minutes, and then rinsed three times with sterile distilled water. Then, they were put on plates with water-agar to germinate in the darkness for 48 hours. After this period they were treated according to the treatments described in Table 1; 20 seeds were used in each treatment. The two antimitotic agents used were colchicine (Sigma-Aldrich, usa) and oryzalin (Duchefa, Holland) dissolved with 1% of dmso; all solutions were sterilized by filtration.
Table 1: Concentration of antimitotic agents and exposure time used in in vitro (ie) and seed (se) explants.
| Product | Concentration | Time | ID(*) |
|---|---|---|---|
| Oryzalin | 2.88 µM | 24 hours | T1 |
| Oryzalin | 2.88 µM | 24 hours | T2 |
| Oryzalin | 57.6 µM | 24 hours | T3 |
| Oryzalin | 86.4 µM | 24 hours | T4 |
| Colchicine | 250 µm | 24 hours | T5 |
| Colchicine | 500 µm | 24 hours | T6 |
| Colchicine | 751 µm | 24 hours | T7 |
| Oryzalin | 2.88 µM | 48 hours | T8 |
| Oryzalin | 28.8 µM | 48 hours | T9 |
| Oryzalin | 57.6 µM | 48 hours | T10 |
| Oryzalin | 86.4 µM | 48 hours | T11 |
| Colchicine | 250 µM | 48 hours | T12 |
| Colchicine | 500 µM | 48 hours | T13 |
| Colchicine | 751 µM | 48 hours | T14 |
(*)Treatment identification
After applying the treatments, all explants (ie and se) were rinsed three times with sterile distilled water and grown in vitro. Treatments were repeated four times under the same conditions in independent experiments, including one clone per trial. After treatments with antimitotics, 0.2% ppmTM (Plant Preservative Medium, Plant Cell Technology, usa) was added in the culture medium before autoclaving.
The explants were placed in the growth chamber at 21°C (+/-2°C) and were kept in the dark for the first five days to prevent oxidation. After this period, growth was carried out with a light intensity of 15 umol.m2-1. s-1, and a photoperiod of 16 hours of light.
2.1.1 Chloroplast count
The technique of observing and counting chloroplasts in the stomatal guard cells was optimized to have an approximation of the outcome of duplication treatments. A Nikon fluorescence microscope (Optihot-2 model HB 10101AF, Japan) was used. The chloroplasts were counted on the underside of the leaf, in 20 cells on five different fields and the average was calculated with a magnification of x400. Data were analyzed by simple variance analysis, comparing the means by the Tukey test (P<0.05%).
2.1.2 Flow cytometry
Plants that differed statistically from the control were selected for analysis with the flow cytometer. Determinations of the ploidy level were carried out in the Flow Cytometry Service of the Institute of Biological Research Clemente Estable (secif-iibce). The ploidy analysis was carried out in accordance with Doležel and Göhde22. Small pieces of young leaves (60-100 mg) of each sample plant were cut using a sharp blade and placed in a petri dish containing 0.5 ml of frozen Otto I buffer solution (0.1 M citric acid + 0.5% Tween 20). It was filtered through a 50 µm nylon mesh membrane directly into a 5 ml (12 x 75 mm) cytometry tube and kept at room temperature for at least 60 minutes. After adding 0.5 mL of Otto II buffer (Na2HPO4.12H2O 0.4 M) and propidium iodide (PI, 50 µg mL-1), the plant cell nuclei were analyzed by fc. A diploid sample of E. dunnii was used as an external reference ploidy pattern. Ploidy determinations of fc were performed on a MoFlo Astrios eq flow cytometer (Beckman Coulter, usa) equipped with a 200 mW 488 nm laser. The cytometer was calibrated using 3.0 µm Ultra Rainbow fluorescent particles (Spherotech, usa). The fluorescence emitted by pi was detected using a 576/21 band pass filter. The following parameters were analyzed: forward dispersion (fsc-height with mask P1), lateral dispersion (ssc-height), area 576/21 (fluorescence intensity vdg), and width 576/21. The doubles were excluded using dot graphs of 576/21 pulse area versus 576/21 pulse width.
2.1.3 Cloning of plants identified as polyploids by flow cytometry (fc)
For the cloning of polyploid plants, the same culture medium described to introduce and multiply in vitro explants was used. Similarly, subcultures were made every 15 or 20 days. When cloned explants exceeded 100, they were moved into the rooting medium. At this stage, the ms medium (1962), reduced to the third part in its mineral salts was used, and indole butyric acid 2 µM was included. Plants remained in this environment for a month and then acclimatized in a greenhouse at 20°C (+/-5°C), and high relative humidity. Peat moss (Tref.P4), mixed with inert perlite in a 3:1 ratio, was used for acclimatization. The plants were covered with nylon and 80% shade mesh to prevent dehydration and sunburn, for the first 15 days. After this period, plants were ventilated gradually, up to 40 days. After this period, coverage was no longer used.
3. Results and discussion
In colchicine treatments, T14 was the only one that induced solid polyploids in se, despite presenting very high toxicity; with the lowest concentrations and the longest exposure time, T12 and T13 showed chimeras and plants reverted to diploid level during acclimatization (table 2). The colchicine caused damage to both types of explants, being greater in ie than in se. Conditions of treatments T12, T13 and T14 were lethal for ie and in these treatments no individuals were recovered. Treatments T5, T6, T7, which were temporarily shorter, were also highly phytotoxic when applied on ie and to a lesser extent also affected se (Fig. 1).

Figure 1: Toxicity of colchicine in a) apical necrosis in germinated seed (se); b) in vitro explants treated with colchicine (ie).
The higher concentration of colchicine (T7), the damage over ie increased. In contrast, se tolerated the most aggressive treatment.
Table 2: Summary of treatment results in polyploid induction.
| Explant | Treatment | Result | Observations |
|---|---|---|---|
| SE | T1 to T4 | - | Low toxicity |
| T5 to T7 | - | Average toxicity | |
| T8 to T11 | - | High toxicity | |
| T12 and T13 | Mixoploidy | High toxicity | |
| T14 | Polyploid | Very high toxicity | |
| IE | T1 to T4 | - | Low toxicity |
| T5 to T7 | - | High toxicity | |
| T8 to T10 | - | High toxicity | |
| T11 | Polyploid | High toxicity | |
| T12 to T14 | - | Very high toxicity |
For the ie, only oryzalin in the highest concentration and exposure time (T11) induced polyploids in three of the four clones introduced in vitro. In both explants used (ie and se), bacterial contamination was observed (Fig. 2). ppmTM was used during three or four subcultures in clone treated individuals. No contamination was observed in the control treatment explants.
Stomata observation was performed directly on the underside of the leaf in the two types of explants. Staining with fda made fluorescent chloroplasts clearly visible, which facilitated counting, both in the x200 or x400 magnification. In control plants, the number of chloroplasts was 5.5 on average in the stomata cells; on the other hand, in duplicate plants, the observed number was higher, in some cases almost doubled the number of chloroplasts present in control plants (Fig. 3a).
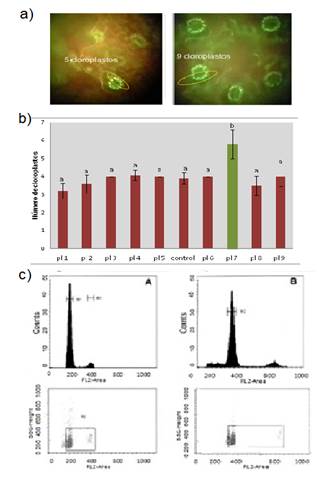
Figure 3: Comparison between explant treatments: a) Observation of stomata in the fluorescence microscope (left control plant, right treated plant); b) chloroplasts count in the stomatal guard cells in diploid plants and in candidate plants (right). Different letters indicate difference of means, Tukey, P<0.05%; c) identification of polyploids in the flow cytometer.
The chloroplast number indicator allowed increasing the number of polyploid plants confirmed by fc from 3% to more than 40%. Significant time in the use of the cytometer was saved, due to this approach. As Figure 3c shows, when the nuclei of 4x polyploid cells passed in front of the laser, the fluorescence emission was higher (channel 400) compared to the control (channel 200). Figure 3c shows a small peak to the right of the main peak, both in the control plants as in the duplicates, corresponding to the cells in stage G2 of the cell cycle. All identified polyploids corresponded to level 4x, tetraploids. In the in vitro cloning stage of tetraploids, the response to growth in vitro was variable depending on the individuals. The first four subcultures included 0.2% ppmTM. In the case of ie (clones), a high percentage of rooted plants was obtained, in contrast, great variability was observed in se, from 2% rooting to some individuals that rooted in more than 80% in the culture medium used. The acclimatization process lasted two months in most cases. After this period, plants continued their growth until reaching a height of 20 to 30 cm for field installation. The process lasted two years from the in vitro introduction of explants to the production of acclimatized duplicate plants. The duplication efficiency for the two antimitotics used did not exceed 5% of the explants processed in any treatment.
In polyploid induction, several factors influence positive results, the most determinant are the antimitotic agent, concentration, and exposure time23. Effective treatments with positive results are different depending on the type of explant used. The agent that induced polyploids in ie, was oryzalin. However, one of the four clones used did not double in T11. This clone suffered greater phytotoxicity in exposure to oryzalin; in this case, another combination of factors should be evaluated: concentration/time to generate tetraploid individuals. Colchicine induced lethality in this clone in most treatments; antimitotic agents could be included in the culture medium to decrease direct contact of explants with these, but increasing exposure time. Colchicine is used in higher concentrations than oryzalin, since it has a low affinity with some plant species. It is more toxic and increased concentrations cause the death of plant cells due to the blockage of spindle fiber development during mitosis24. In all colchicine treatments, explants had a high level of necrosis and damage; it was very toxic, but effective in se duplication. The se were more vigorous as explants and tolerated better treatments. Mortality was associated with product concentration, as observed by other authors25. High concentrations of colchicine are necessary because of its low affinity with plant cells5, but this is not tolerated by some plants.
According to the results, the only effective treatments in inducing polyploids at E. dunnii were: T11 and T14, both with the highest product concentration and longest exposure time. dmso (dimethylsulfoxide) was used in both cases to dissolve compounds26. Oryzalin is not soluble in water and dmso must be included to obtain the solutions. In contrast, colchicine is very soluble in water, but dmso increases its effectiveness, by increasing permeability in tissues to reach the nucleus of the plant cell5. The dmso is also phytotoxic, so its effect is added to that of the antimitotic agents, but its use cannot be excluded in experiments5. The use of explant type (ie or se) affects the expected product of polyploid materials. When ie are used, variability is expected due to the doubling of the allelic dose of the starting material. The allelic dose may affect the genetic expression and therefore the phenotype. Regarding se, its variability derived from recombination of the original gametes is added and, therefore, in this case, the expected variability is potentially greater. This affects the breeding program of the species under consideration, while the polyploids generated can be used for direct evaluation or as a source of future parents, as long as the polyploid fertility is not affected.
The chloroplast count was useful in reducing the number of samples sent to the cytometer. This resulted in significant time and input savings. Although the chloroplast count was useful, it is necessary to confirm the ploidy through fc; this technique is a non-destructive powerful tool and only requires small portions of tissue for evaluation27. It has a large analysis capacity, evaluating more than 5000 cell nuclei per sample, and hundreds of samples in a short period of time16. The application of the in vitro model allowed the use of small volumes of antimitotic agent solutions of less than 20 mL, this enabled an easier manipulation of discard solutions. Another advantage of in vitro cultivation was the rapid obtention of duplicate plants, which determined that the in vitro system is preferred over in vivo applications in chromosomal duplication treatments5. Over a two-year period, 50 or more plants were obtained from the three duplicate clones and from the seed individuals; with this result, field comparative tests were established, among the polyploid materials to be evaluated for their production characteristics. In addition to the short time for plant generation, another advantage of using the in vitro model is the possibility of discarding chimeras early and eliminating plants that will then revert to the diploid state28.
4. Conclusions
The most relevant general findings of the research "Polyploid induction of Eucalyptus dunnii Maiden to generate variability in breeding programs" are the following:
Although the duplication efficiency with the two antimitotics was of 5% maximum, all identified seed and in vitro polyploids corresponded to the tetraploid level.
















