Introduction
Olive production in Uruguay has increased recently, reaching nowadays around 10,000 ha. Olive cropping has been well adapted in several countries in South America, both for olive and oil production. In these countries, pest damage could be of significant expression if control measures are not provided1.
The most important olive pests for olive production, Bactrocera oleae and Prays oleae, are not present in South America, where the scale Saissetia oleae, the olive moth Palpita spp., and the eriophyid mite Oxycenus maxwelli are cited as the main olive pests2. In southern Brazil, the moth Palpita forficifera and the scales S. oleae and Saissetia coffea were reported as the main pests, in addition to some hemipteran and two thrips species3.
In Uruguay, the moth P. forficifera, the H scale, S. oleae and Hylesinus oleiperda (Coleoptera: Curculionidae) are reported4. The presence of the white scale Aspidiotus nerii, the Australian red scale Aonidiella aurantii, and the curculionid Phloeotribus scarabaeoides are also cited5. P. forficifera was registered in 2003 surveys conducted in the north-western region of Uruguay. Nowadays, it is a common pest all over the country6. First moths appear in early spring and the flights are common during summer and fall. Larvae are present since late spring to mid-autumn5. For moth surveys in olives, non-traditional black light traps have been developed7, or sexual pheromones8 or a combination of both methods is used9. Sex pheromones have been successfully employed as a sexual confusion method to control P. oleae10.
The eriophyid mites Oxycenus maxwelli, Ditrymacus athiasellus and Aceria oleae are present in Argentina, Brazil and Chile11. Their main damage is fruit deformation in adult trees and blooms and leaves deformation in seedling trees. The importance of these mites has been associated with some cultural practices, such as irrigation, nitrogen fertilization, higher planting density and broad spectrum insecticides.
Black scale, or H scale, is the most extended hemipterous pest in olives, always associated with fumagine (Capnodium leaophilum), which grows over this insect honeydew. In southern Uruguay, two generations of this scale were registered, one in spring and the other in fall12. Thus, this study was aimed at surveying the species of olive moths and mites present in an olive orchard in eastern Uruguay.
Materials and Methods
A survey was conducted in an olive 50 x 100 m plot, in Rocha, Uruguay, in the reproductive period of olives, between November 2016 and April 2017. Between November 2016 and February 22nd, 2017, no pesticide applications were made in the area, when one application of insecticide was needed for the control of lepidopterous larvae.
For collecting adult lepidopterans, three adapted black (UV) light traps7 were installed in between the olive trees in one of the rows, with a distance of 30 m between one trap and the following. The cultivars present in the survey plot were Arbequina, Coratina, and Picual. Traps were energy-supplied by a battery connected to a pair of solar panels, controlled by a timer device programmed to work every two nights, previous to samples collecting. The samples from these traps were collected also on a weekly basis, from December 2016 to March 2017.
For the mites and lepidopteran larvae survey, every weekly sampling date, four new sprouts in three replicates were randomly detached from each cultivar. These were maintained and examined at the Laboratory of Animal Biology of Centro Universitario Regional del Este, under a binocular stereomicroscope (40x) for searching mites. In these samples the number of sprouts with presence of eriophyid mites and their damages were registered. Also randomly obtained larvae of Palpita sp. were collected and reared in laboratory conditions, to obtain the adult stage for their identification. For monitoring the presence of larvae in the foliage, weekly samples were taken with a portable vacuum aspirator, during one minute in each olive tree, around their canopy, in three replicates per cultivar. Labeled samples were placed in plastic bags (aspirator) or in hermetic plastic vials containing ethyl alcohol 70 % (light traps) and maintained in a refrigerator until being analyzed.
The eriophyid mites were identified at the Laboratory of Plant Quarantine, Embrapa Genetic Resources and Biotechnology, Brasilia, Brazil. They were mounted in permanent microscope preparations using Berlese modified medium13 and then identified using a phase contrast microscope14. Specimens of mites collected in this survey were deposited at the Reference Mite Collection of the Laborator of Plant Quarantine.
Adult specimens of Palpita sp. collected with the blacklight traps and those emerged at the laboratory from a stock mass larvae rearing from the field were identified by Dr. Vítor O. Becker, at Instituto Uiraçu, in Camacan, Bahia, Brazil, where the genitalia remained.
To assess if there were differences on the abundance of Palpita adults among cultivars, we ran GLM models with negative binomial error distribution and logit link function. Similarly, GLM with binomial error distribution and log link function were used for assessing if there were differences among the olive cultivars in the probability of occurrence of eriophyid mites. Contrasts method was applied for assessing which cultivar (i.e. levels of the factor variable) had a significant effect on Palpita abundance, as well as on the occurrence of the eriophyid mites15. We ran models with R 3.3.116 to run the GLMs.
Results and Discussion
A total of 164 adults of Palpita sp. were captured in blacklight traps from January 2017 until April 2017, reaching a maximum by the end of February and beginnings of March. With the portable vacuum aspirator, we collected, in total, 178 larvae of the olive moth. That sudden increase in the number of adults collected led the responsible of the crop management to make an insecticide application (Figure 1). It is assumed that such intervention might have been responsible for the later decrease of adults captured.
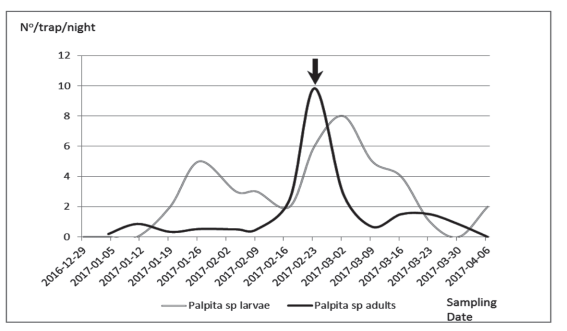
Figure 1: Mean number of Palpita sp. adults (by night, collected in blacklight traps) and larvae (by sample, collected with a portable vacuum aspirator), from December 2016 to April 2017. The arrow indicates an insecticide application.
All specimens sent to Dr. Vítor O. Becker were identified as Palpita persimilis Munroe, 1959 (Lepidoptera: Crambidae, Spilomelinae). This is the first report for this species in olives in Uruguay. This species has been cited for Chile17)(18 and Brazil19. Palpita persimilis is sympatric with Palpita forficifera in southern Brazil (Becker, V. pers. comm.), which is probably the same status these two species have in Uruguay, as the latter has been already cited4)(5)(6.
It must be pointed out that P. persimilis and P. forficifera are morphologically identical, the moths having almost entirely white wings, with a wingspan from 19 to 27 mm. The labial palpi of P. persimilis are mostly reddish brown with distinct dark-brown to black scales bordering the reddish area dorsally and ventrally; the same dark scales are also prominent on the maxillary palpi and lateral margin of the frons. Their identification must be always confirmed by the study of their genitalia. Males of P. persimilis have elongate processes on the valvae that curve around to parallel the distal margin of the valva. Each process has two parts. The inner parts are symmetrical and have swollen grooved apices. The outer parts are narrow and asymmetrical, with the right-hand one nearly as long as its inner part and slightly bifurcate, and the left-hand one half as long as its inner counterpart. Females have lamella postvaginalis asymmetrical, tapered like a spout20.
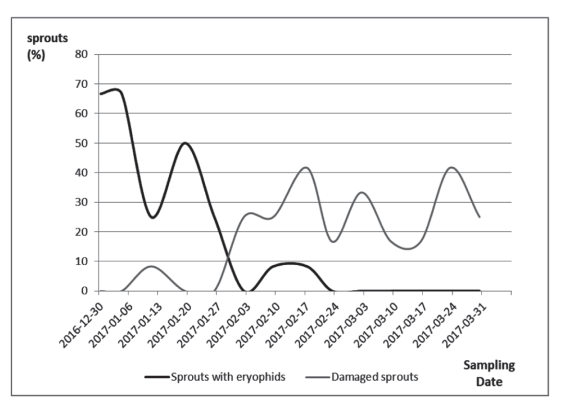
Direct examination of new olive sprouts showed an eriophyid species as the predominant mite, and its presence on the sprouts declined from December to February, since when were almost not found (Figure 2).
In the conditions of this survey, the presence of eriophyid mites was detected in most of the new sprouts examined in the earlier sampling dates, in summer, and the incidence decreased as the sampling period went forward. In this case, the number of mites had already decreased significantly before the insecticide application (February 22nd). Mite damage is produced to the buds and it is expressed and easily verified when the leaves of the sprout expand. Then, the damage stays in the sprout even when the mites are not there, so the damage level did not decrease towards the final sampling dates.
The Eriophyid mite species was identified as Oxycenus maxwelli (Keifer)21 (Acari: Eriophyidae), also representing the first report for this species in olives in Uruguay. These mites are exclusively phytophagous and cause leaf silvering and deformations in most plant tissues, except for roots. Oxycenus maxwelli had been cited in Argentina22, Chile and Brazil23)(24. This species was described from California, USA, as a mite with a fusiform body, orange in color, females measuring 140-160 ìm21. Opistosoma (dorsal region) has a serrate median elevation, arrows of dorsal shield near the posterior margin and some dorsal rings have lateral projections, frontal lobe acuminate, rounded above24. The female genital shield has 18-20 longitudinal ridges21.
According to GLM model, Palpita persimilis larvae abundance was significantly higher on cv Arbequina than on Coratina and Picual (Z = 2.389, p = 0.01), while no differences were found between these latter (Z = -0.935, p = 0.349) (Figure 3).
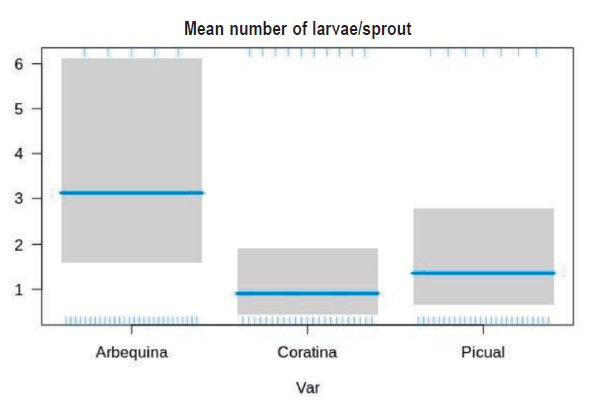
Figure 3: Adjusted GLM for Palpita persimilis larvae abundance in three cultivars. The blue line indicates the mean, while 95 % confidence intervals are shown in grey.
No differences were found for the occurrence probability of the mite O. maxwelli between cultivars (Figure 4). GLM on probability of occurrence of O. maxwelli showed no significant differences either between Arbequina and Coratina and Picual cultivars (Z = 0,105, p = 0,916) and Coratina and Picual (Z = 1,450, p = 147; Figure 4).
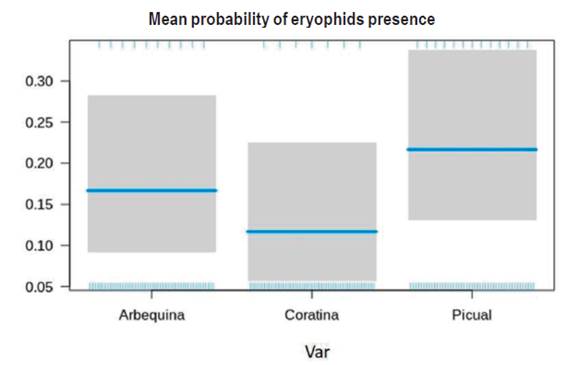
Figure 4: Adjusted GLM for the probability of the presence of eriophyid mite Oxycenus maxwelli on new olive sprouts of cultivars Arbequina, Coratina and Picual. The blue line indicates the mean, while 95 % confidence intervals are shown in grey.
The portable vacuum aspirator provides an active useful sampling method for a general capture of canopy inhabitant organisms. However, this sampling method misses those arthropods that have nocturnal habits. This fact was, to some extent, complemented by the samples taken with the light traps. However, in the present study, light traps were used only for monitoring the lepidopteran adults defined as one of the target organisms.
Final Considerations
Palpita persimilis Munroe (Lepidoptera: Crambidae) and Oxycenus maxwelli Keifer (Acari: Eriophyidae), are reported for the first time in an olive crop in Uruguay, and appear to be key pests of this production in the south-eastern region of this country.
However, the incidence of these species in terms of damage or economic impact was not studied as it depends on the interaction of different factors that affect their presence/abundance (climatic conditions, natural control, use of agrochemical products, among others).
The sampling methods showed to be adequate for the general characterization of the arthropods present in the area and could be useful for monitoring main target species.