Introduction
Swine product quality refers to the composition characteristics of the carcasses, determined by the proportion of the different deposited tissues and the composition of those tissues, involved in carcass and meat quality concepts1)(2.
Quality concept varies based on the perspective of each link in the production chain. For the producer, it is associated with lean tissue content, growth rate and feed conversion ratio; the consumer values sensory aspects, physical appearance, hygienic quality and preparation and use simplicity3; the industrial sector considers fat color and consistency, which determine its stability and preservation capacity4)(5; while nutritionists emphasize the aspects associated with the nutritional quality of the product, especially those related to lipid composition and its relationship with cardiovascular health5)(6)(7.
Lipid content of pork is very variable, between 1, 5 and 13%, being determined by food, sex, breed and slaughter weight5)(6)(8. The intramuscular fat, or marbling, is associated with the tenderness, juiciness and aroma of the meat, considering that for optimum organoleptic quality the meat should have between 2 and 2.5% of intramuscular fat3. Sensory attributes, such as taste and tenderness, are adversely affected below 2%, while nutritional quality is affected above 3.5%9. Capra and others10 conclude that commercialized pork cuts in Uruguay, except for bondiola, can be classified as lean meats (<10% fat content) or very lean (<5% fat content).
From the industrial point of view, firm fats, with a high degree of saturation and greater resistance to rancidity are attempted. Fat firmness is directly related to the ratio between saturated fatty acids (SFA) and polyunsaturated fatty acids (PUFA): fats are solid at room temperature when there is a high proportion of SFA and as the degree of unsaturation increases, the melting point decreases to liquid at room temperature8. Stearic (C18:0) and linoleic (C18:2) fatty acids have a high correlation with fat consistency, being the ratio (18:0):(18:2) used to define the degree of firmness5. Daza and Buxadé4 consider a fat soft when the stearic:linoleic ratio is lower than 1.47. For its part, regulations of the Parma Consortium establish an upper limit of 15% linoleic acid in fresh hams.11
In monogastrics, part of the fatty acid intake is deposited directly in the tissues, therefore, the lipid profile of the diet is reflected in the composition of body deposits12)(13. There is also dependence on the genetic type, since high fat content genotypes tend to have a higher SFA and a lower UFA proportion13, while lean genotype pigs have less fatty acids a novo synthesis. The lipid composition of these carcasses reflect to a greater degree the dietary fat composition8)(14. In surveys carried out in Uruguay, it is concluded that the lipid profile of commercialized pigs has low levels of SFA, while high and very variable levels of monounsaturated and polyunsaturated fatty acids respectively, with high dependence on the type of food received5)(15.
In humans, the saturated fatty acids intake raise the concentration of low density lipoproteins (LDL), associated with the occurrence of coronary diseases. When PUFA replace SFA, LDL are reduced, but high density lipoproteins (HDL) also decrease, while MUFA reduce LDL levels without modifying HDL levels. Most PUFA are grouped into two series: the ω6 series that includes linoleic, essential fatty acid, and the ω3 series, which includes linolenic acid16. The challenge in pork production is to reduce meat SFA content and increase the amount of monounsaturated (oleic-omega 9) and polyunsaturated fatty acids (linoleic-omega 6 and linolenic-omega 3), which are associated with cardiovascular health benefits6)(15.
Meat of pigs on maize-based diets has a higher content of linolenic acid, compared to diets based on other cereals, which is favorable to human health, but associated with a higher risk of alteration due to rancidity and lower industrial quality, while the oleic acid content decreases6)(16.
In the case of sorghum, although it contains10% less lipids than maize17, 83% of them consist of unsaturated fatty acids, of which 56% is linoleic acid, which makes pigs on diets based on sorghum generate fats with higher content of monounsaturated and polyunsaturated fatty acids, mainly linoleic and oleic acids, with respect to diets based on maize6)(16.
Numerous studies maintain that the substitution of soybean meal for canola meal in the feeding of finishing pigs does not affect carcass characteristics, as long as the same levels of digestible amino acids and the energetic concentration of the diet are maintained18)(19)(20)(21)(22)(23)(24. On the other hand, Caine and others25 observed that the carcasses of pigs on diets with canola expeller had a higher content of intramuscular fat or marbling.
Canola oil is rich in MUFA, n-6 and n-3, highlighting its linolenic acid content26. Diets with expeller or canola meal increase the MUFA and C18:3 content, decreasing the proportion of C18:6 in pigs fat, reducing the n-6:n-3 index and the fat melting point. As a consequence, its industrial value is reduced, although the quality from the nutritional point of view is improved13.
No differences were observed in carcass characteristics of pigs on diets where soybean meal was replaced by forage peas, supplemented with synthetic amino acids, compared to control diets based on maize-soybean meal27)(28)(29 or barley-soybean meal30)(31.
Regarding the composition of body lipids, Thacker and others27 and Chrenková and others31 observed that diets where peas replaced soybean meal increased the ω3 fatty acid content and decreased theω6 of dorsal fat, which significantly reduced the ω6:ω3 ratio, considered beneficial for consumer’s health.
The general objective of this study was to determine the effect of the use of sorghum, canola and pea expeller in fattening pigs diets on the quality characteristics of carcass and lipid composition of body fat.
Material and methods
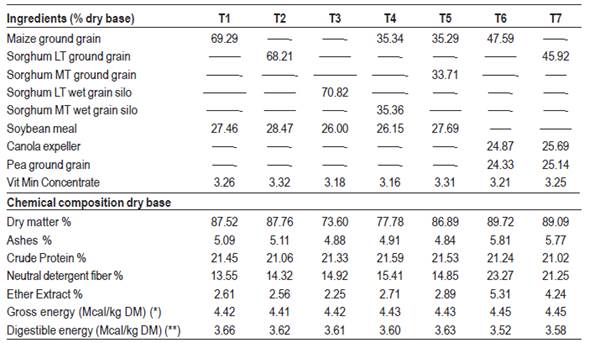
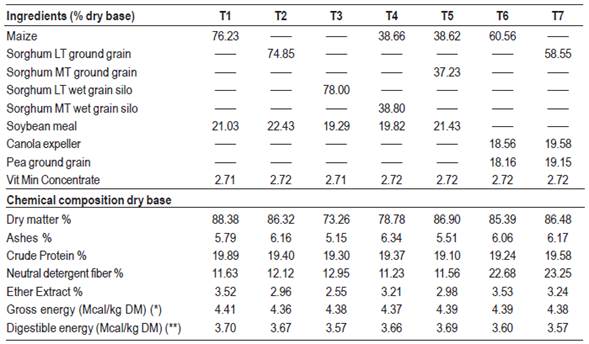
Carcasses from pigs that during the fattening period received the following experimental diets were evaluated: T1 (control): maize/soybean meal; T2: Low tannin sorghum grain, dry ground/soybean meal; T3: Low tannin sorghum wet grain silo/soybean meal; T4: (Maize + sorghum with medium tannin content wet grain silo)/soybean meal; T5 (Maize + medium tannin content sorghum grain, dry ground)/soybean meal; T6: Maize/(canola expeller + peas); T7: Low tannin sorghum grain, dry ground/(canola expeller + peas).
Experimental conditions during fattening period
The experimental protocol was approved by the Ethics Committee on the Use of Animals, endorsed by resolution of the Council No. 1546, Agronomy College. Pigs were housed in the Porcine Testing Station of the Sayago Farm of the Agronomy College. From the beginning of the fattening period (35 ± 1.5) until they were sent to the slaughter plant, animals were housed in individual pens, with a front feeder pan, with ad libitum access to drinking water by means of automatic pacifier-type troughs.
Animals
The carcasses from the slaughter of 42 castrated male pigs were evaluated. They were assigned at a rate of six animals per treatment that had received the experimental diets in the period from 35 to 105 kg of live weight. The pigs were of uniform genetic type due to a terminal cross formed by the mating of mothers of Large White x Landrace crosses and a boar of Landrace x Pietrain cross.
Experimental feed
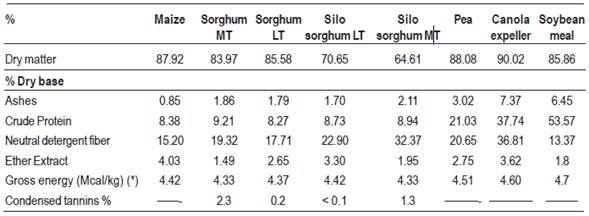
Diets were formulated according to the recommendations of NRC32 for fattening categories. Two diets were used for each treatment depending on the growth phase of the animals (growing or finishing), using the same food, varying the proportions in each stage to adjust to the nutritional requirements. Rations were supplied at will throughout the experimental period and the same diet was continued until the animals were transferred to slaughter. Tables 1, 2, 3 and 4 show, respectively, the chemical composition of the foods used, the percentage and chemical composition of growing and finishing diets, and the lipid content and lipid composition of the diets used in the finishing period.
Chemical analyzes of the food and experimental diets were carried out in the Animal Nutrition Laboratory of the Agronomy College. Determinations of tannin content in sorghums were conducted in the Grain Quality Laboratory of INIA-La Estanzuela. The lipid content and the lipid profile of the diets were determined in the Fats and Oils Laboratory of the Chemistry College.
Slaughter and carcass evaluation
As the animals reached the weight corresponding to the end of the behavior test (108.51 ± 2.34 kg live weight) they were sent to slaughter in the ARDISTAR slaughter plant, located in La Tablada, Montevideo. A 24-hour fasting was carried out prior to the slaughter by ceasing the food supply from the day before the planned transfer to the slaughter plant. The slaughter was performed by following the routine procedure used in the industrial plant, previously desensitizing by electrocution, subsequently exsanguinating by sectioning arteries and veins of the brachiocephalic trunk, continuing the slaughter line with blanching, peeling and eviscerating. In the final stage of the slaughter process, after the head is separated, the carcasses are cut longitudinally in two half carcasses, which are moved to a cold chamber.
Carcass measurements and sampling
Measurements were taken at the end of the slaughter line, before entering the cold chamber, following the procedure used by Capra and others7. Carcass length (from the front edge of the first rib to the center of the pubic symphysis) was measured with a measuring tape on the hanging left half-carcass, as well as dorsal fat thickness (average of the measurements on the dorsal midline at the points corresponding to the last rib and gluteus medius muscle).
A cross section was made on the left half-carcass at the level of the last rib, including the loin eye, the bone and the subcutaneous fat included in the steak or «pork rib» according to its commercial definition. The exposed area of this cut was traced so as to determine the area.
Samples of subcutaneous fat and longissimus dorsi muscle were removed on the dorsal line of the left half-carcass, at the level of the last rib, being frozen and sent to the laboratory to determine lipid profile and intramuscular fat content, respectively.
Meat quality characteristics
By scanning the areas traced in the slaughter plant and applying the area determination program IMAJE J33, the loin eye area (muscle longissimus dorsi), corresponding to the subcutaneous fat included in the steak, and the meat:fat ratio in the steak were established.
The intramuscular fat content of the longissimus dorsi muscle samples extracted in the slaughter plant was determined. This was conducted in the Fats and Oils Laboratory of the Chemistry College, by quantitative extraction of the intramuscular lipids of the meats by Folch Method34.
Lipid profile determinations
They were carried out in the Fats and Oils Laboratory of the Chemistry College using gas chromatography. The lipids extraction was done at room temperature with hexane:isopropanol in a 3:2 ratio. The extracted fat was derivatized according to the IUPAC 2.301 technique to obtain the methyl esters and then the analysis was carried out by gas chromatography (according to AOCS Ce 1c-89, AOCS Ce 1f-96 technique). A Shimadzu equipment model 14B was used, equipped with a Supelco SP-2560 capillary column.
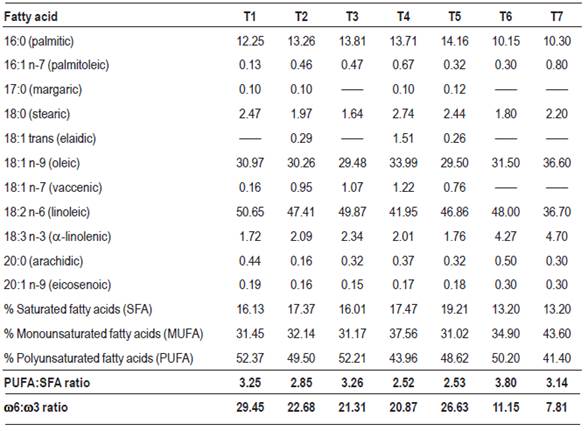
From the analysis of the dorsal fat samples, the relationships between saturated, monounsaturated and polyunsaturated fatty acids, expressed as a percentage of total fatty acids, were evaluated; and the concentration of stearic, oleic, linoleic and linolenic fatty acids was studied for each of the feeding systems evaluated.
Parameters evaluated
With the slaughter data, the following carcass characterization indicators were evaluated: carcass length (cm); dorsal fat thickness (mm); eye loin area (cm2) and meat:fat ratio in the steak (%).
From the lipid profiles of the dorsal fat samples, the following were evaluated: % of saturated fatty acids (SFA);% monounsaturated fatty acids (MUFA); %polyunsaturated fatty acids (PUFA); PUFA:SFA ratio; concentration of stearic, oleic, linoleic and linolenic fatty acids expressed as a percentage of total fatty acids; ω6 and ω3 concentration; ω6:ω3 ratio.
Model and statistical analysis
A random parcel design was applied, with the observation unit being each carcass. The adjusted model corresponds to a random variable with normal distribution, with the following general formula:
yin = µ + Di + εin
where: yin is the response variable; Di the effect of the received diet;εin the experimental error.
Results were analyzed by means of the F test with precision levels of 1 and 5%, performing, in case of finding significant differences, the means comparison using the test of least significant difference (LSD) at the same significance levels.
Results and discussion
The carcasses corresponding to treatment 5 (substitution of 50% of maize by dry ground grain of high tannin sorghum) could not be evaluated due to reasons related to the trial logistics. However, it was possible to obtain and analyze the dorsal fat samples for this treatment, therefore, it is still included in the study description.
Table 5 shows the characteristics obtained for the carcasses studied. No significant differences were observed in the carcasses length when maize was replaced by low tannin sorghum, presented as ground grain or as wet grain silo, using soybean meal as the only protein supplement. The same result was observed when 50% of the maize was replaced by wet grain silo of medium tannin sorghum. When all of the soybean meal was substituted for a combination of canola expeller and peas, there was no difference in length compared to the rest of the diets when the cereal was low tannin sorghum. However, when maize was used combined with canola and pea expeller, the carcasses were shorter than those of animals fed with T1. This result differs from what Maupertuis and others19 and Chrenková and others31 observed and cannot be attributed to differences in the animals genetic potential as mentioned by Campion3, since all of them are from the same origin and time. Observations indicate that the animals of treatment 6 had a longer adaptation period to start a regular food intake, which could have affected the initial development, resulting in shorter animals.
Carcass dorsal fat thickness was similar between treatments. Observed values are greater than the obtained by other authors such as Braun and others14 and Silva and others35 in Argentina or Bauza and others36 in Uruguay, who mention values ranking from 20 to 26 mm. Nevertheless this observed thickness is smaller than the mentioned by Barlocco and others37 in animals with Pampa crosses or by Dobao and others38 for Iberian pigs; being very similar to what was reported by Petrocelli and others39 in its study of characterizing the carcasses received in the slaughter plants in Uruguay. Aspects associated with the animals’ genetic potential and the free feeding system used in this trial may be the cause for the observed subcutaneous fat content.
Results from the loin eye area of carcasses from T1 were similar to those reported by other authors3)(40 for pigs with similar genetic characteristics. No differences were found for this indicator between T1, T2, T3 and T6, being significantly smaller (p ≤ 0.01) in carcasses from pigs fed with wet grain silo of medium tannin content sorghum substituting 50% of maize supplemented with soybean meal (T4) and also from pigs fed with diets based on low tannin sorghum, supplemented with canola expeller and peas (T7). The loin eye area is very important for the carcass value when destined to fresh consumption, since it is negatively correlated with the subcutaneous fat content, considered as undesirable, and determines the useful percentage of the «pork rib», as shown in Table 5. As pointed out by Hurnik40 the loin eye area is determined by the genetic potential of the animals and likewise its expression depends on the protein balance of the diets, mainly on the available lysine content. The smaller loin areas obtained from diets based on silo of medium tannin sorghum would be associated with the lower digestible protein content of this diet. Moreover, we observed that all diets that contain sorghum grain substituting maize present smaller loin eye area, which would be related to the lower bioavailable lysine content of this cereal41)(42. The substitution of soybean meal for the mix of canola expeller and peas did not affect the loin area in the based maize diet, while in the combination with LT sorghum the result was lower, which allows us to infer that the effect was due to cereal and not to the substitution of soybean meal. Regarding fat area and meat content percentage in the steak, the values follow the same trends as the loin eye area. It is important to highlight the fact that these values are considered to determine the consumer’s performance «on the plate».
The intramuscular fat content was significantly higher (p ≤ 0.01) in the cattle from T4 and T6 diets. The observed value for T4 treatment is above the maximum admitted value from the point of view of nutritional quality mentioned by Fernández and others9. In the other treatments the intramuscular fat content was within the range considered desirable both for fresh consumption and for its industrialization by Echenique5 and Campion3. The high content of intramuscular fat observed in T4 can be associated with an imbalance in the digestible energy:digestible protein ratio, given the lower use of the protein of sorghum with a medium tannin content.
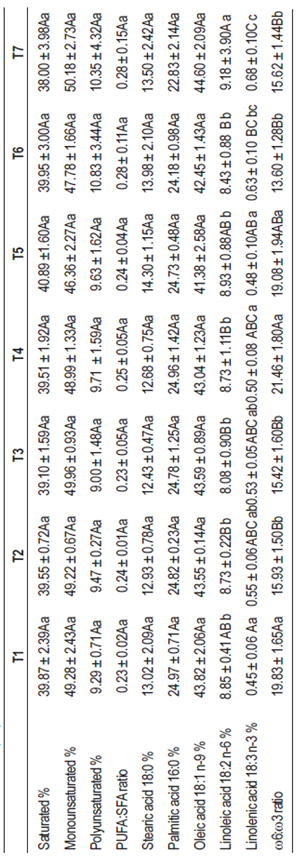
Table 6 shows the lipid profile results of the dorsal fat of cattle from the different treatments. No differences were detected between diets in the saturated, monounsaturated and polyunsaturated fatty acids content, although differences were observed (p ≤ 0.01) between treatments in the content of ω-3 and ω-6 fatty acids. The canola expeller diets produced lipids with a higher ω3 content, reflecting the higher concentration of linoleic acid in them, in accordance with Bertol and others13 observations. On the other hand, ω6 concentrations are very similar between treatments, also reflecting the lipid composition of the original diet. Considering ω6:ω3 ratio, it is observed that the best ratios between these types of fatty acids are obtained from diets including canola and pea expeller, which is of great interest from the consumer’s health point of view, as established by Bañón and others43 and Apple and others12. Considering that peas have an extremely reduced lipid content, as noted by Bauza and others36, it can be concluded that the canola expeller is responsible for the difference in the body fat composition.
Conclusions
It is possible to replace maize with low tannin sorghum, presented as dry grain or wet grain silo in diets for fattening pigs, without affecting carcass composition or the lipid profile of dorsal fat.
The inclusion of wet grain silo of medium tannin sorghum in substitution of 50% of the maize grain has a negative effect on the muscular development of the carcasses that is reflected in a smaller loin eye area and causes an increase in the intramuscular fat content.
Substituting soybean meal for a mixture of canola expeller and forage peas in combination with maize grain or low tannin sorghum grain does not affect pig’s carcass characteristics.
Pigs on diets where soybean meal has been replaced by a mixture of canola expeller and forage peas in combination with maize grain or low tannin sorghum grain, presented a lipid profile of dorsal fat with higher content of ω3 fatty acids, decreasing the ω6:ω3 ratio compared to diets with soybean meal, which is considered beneficial to consumers’ cardiovascular health.
It is recommended to investigate the use of diets with canola expeller supplemented with synthetic lysine as the main protein source and its effect on the lipid profile of pigs’ fat.