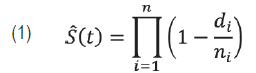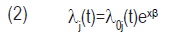Introduction
Passiflora edulis Sims is the main cultivated species in Passifloraceae family. Two formae speciales are known: the purple passionfruit (P. edulis Sims f. edulis) and the yellow passionfruit (P. edulis f. flavicarpa O. Deg.). P. edulis is probably originated in South-America, and the origin of the P. edulis f. flavicarpa is not determined (Pereira, 2008).
As a tropical plant, the passionfruit is well adapted to high temperatures. Low temperatures for a long time cause flower bud and fruitlet drop (Junqueira, 2009). Average annual temperatures between 23 and 27 ºC are ideal for its cultivation (Freitas, 2001). For seed germination, 20 to 30 ºC is the optimum range (Pereira & Andrade, 1994), and in tropical conditions, seeds germinate in three to four weeks (Ferreira et al., 2007; Oliveira et al., 2010).
The yellow passionfruit is the major species of Passifloraceae cultivated in Brazil. It is mainly grown in the tropics. Only 3 % of the cultivated area in 2013 was located in the South region (IBGE, 2015), where the climate is subtropical. In a big portion of that region, planting new orchards during autumn and winter (from mid-March to mid-September) is impracticable because of the risk of frost. However, growing passionfruit nursery plants in greenhouses during those seasons enables the obtainment of bigger plants (compared to the conventional ones) for planting in the spring. This approach increases the yield potential in the first crop (Laredo, 2013). Thus, seedlings production must count on cold protection through greenhouses to be planted at the end of the winter (Carvalho, Stenzel & Auler, 2015).
Passion fruit is usually propagated by seeds. Cloning through grafting and rooting is rarely used because it costs more and delays the process. Protocols for utilization of growth regulators in seeds, aiming the maximization of passionfruit seed germination, are already known. Using gibberellic acid (GA) improves seed germination and a stimulant compounded by kinetin, gibberellic acid, and indole butyric acid promotes initial plantlet growth (Echer et al., 2006; Lima et al., 2009). However, studies in low-temperature conditions have not been published, and it is known that interactions between environment temperature and doses of regulators occur (Malavasi, Dias & Malavasi, 2011). It means that the plant responses to exogenous regulators vary with temperature, and results cannot be widely extrapolated. On the other hand, more than one seed per pot is recommended to be seeded (Meletti, 1996; Siqueira & Pereira, 2001). So, a possibly lower germination rate at lower temperatures could be made up by the seeding of multiple seed sets in a pot. Therefore, the knowledge of the effects of regulator treatments on the growth of the seedlings is important to justify their use.
The objective of this work was to evaluate passionfruit seed emergence and seedling growth, in different sowing dates, and under seed treatments with compounds potentially stimulants of germination, under the conditions of a nursery in the subtropical autumn and winter of western Santa Catarina State, Brazil.
Material and Methods
The work was performed in Chapecó, Santa Catarina State (BRA) 27° 6' 24" S; 52° 36' 48" E hight = 670 m and Cfa climate of Köppen 1 (Wrege et al., 2011). All the experiments were conducted with the cultivar Redondo Amarelo (Isla LTDA) as genotype, from seeds bought in the formal market (seeds sold by a seed company fulfilling all legal requirements). The environment was a greenhouse covered with 10 mm alveolar polycarbonate, concrete ground, anti-aphid net and polyethylene curtains on the sides. Data on air temperature were logged trough a dispositive installed at 1.5 m of height and shaded. Daily degree-days accumulated were calculated according to Ometto (1981), considering 8 and 30 ºC as base temperatures (Veras, 1997).
The experimental treatments were the 12 combinations of four seeding dates (May 2, May 22, June 11 and July 2, in 2013 and 2014), two stimulants and one control. The two stimulants were gibberellic acid (GA) and the mixed compound Stimulate (ST). The GA was applied by immersion of the seeds in water solution of gibberellic acid 1 g L-1 during 96 h before seeding, following Lima et al. (2009), using a water solution of 10 g L-1 of Progibb® (Sumitomo Chemical do Brasil Representações Ltda, Brazil) (gibberellic acid 100 g kg-1). The Stimulate® (Stoller do Brasil LTDA, Brazil) (90 mg L-1 kinetin, 50 mg L-1 gibberellic acid and 50 mg L-1 IBA) was applied directly to the seeds (4 mL kg-1 of seeds) immediately before seeding, according to Echer et al. (2006). The control received no treatment. The whole study consisted of the application of the treatments in three experiments: two in 2013 (Experiments 1 and 2) and one in 2014 (Experiment 3).
In Experiment 1, seeds were sown at the depth of 1 cm in the substrate, in plastic seeding trays with cells of 12.5 mL. The substrate was Tecnomax HF fertilized with 15 % broiler litter (Brugnara, 2014). The trays were kept directly on the ground. In each seeding date, each treatment had three replicates of 80 seeds, 36 in the total. The seeds were observed daily during 40 days after seeding, and the number of plantlets emerged was registered. Data on the percentage of emergence were submitted to a 4 x 3 factorial analysis of variance, followed by a Tukey test for comparisons between stimulants, after a Box-Cox transformation (λ = 2). The effect of seeding date was evaluated by linear and nonlinear regressions. The data on daily emergence were also submitted to a survival analysis. The Kaplan-Meier method was utilized for estimating survival curves and median times to emergence (T50). The function of survival (Equation 1) is estimated empirically by
where di is the number of seeds emerged in a given time and ni is the number of seeds treated (Carvalho et al., 2011). With the objective of formally comparing the treatments, the log-rank test was utilized. It uses the chi-squared statistics to compare observed and expected values in each treatment under the hypothesis of an equal capacity of emergence among treatments. From this assumption called proportional risks, it was estimated the effect of the treatments without making assumptions about the probability distribution of the time for emergence dataset, using the Cox semiparametric model (Equation 2), as it follows:
where λj(t) is the function of risk, λ0j(t) is the base risk, x is the vector of co-variables (treatments) and β is the vector of parameters to be estimated. Cox model is flexible because it does not require the assumption of a particular distribution to the function of base risk [λ0j(t)]. It just assumes that covariables act multiplicatively over the risk, and this is the parametric part of the model (Carvalho et al., 2011). For the adjustment of the Cox model, the control was fixed as the reference for the comparisons. The analysis were performed with the statistical system R (R Core Team, 2014), using the procedures of the Survival package (Therneau, 2014).
Two experiments more (2 and 3) were performed in 2013 and 2014 to evaluate seedling growth. Both were carried out in a completely random design with three replicates, each compounded by four bags in 2013 and six in 2014. Polyethylene, perforated 1.5-L bags (Carvalho, Stenzel & Auler, 2015) were used, filled with Tecnomax HF substrate fertilized with 15 % broiler litter, disposed on the ground. Treatments were the same of Experiment 1. Thirty days after seeding, the plantlets in each bag were thinned leaving alive the biggest. Plants were evaluated for height, leaf number (just the ones with borders separated, except cotyledons), shoot dry matter, collar diameter and leaf area (using a CI 202 leaf area meter - CID-Bioscience, USA), on September 15, which is equivalent to 136, 116, 96 and 75 days after seeding, respectively. Data for each year were submitted to analysis of variance after a Box-Cox transformation (λ = 0.1 for height in 2013 and 2014; λ= 0.1 for leaf number in 2013 and λ = 1 in 2014; λ = -0.2 for collar diameter in 2013 and λ= 2 in 2014;λ = 0.5 for leaf area in 2013 and 2014; λ = 0.1 form dry matter in 2013 and λ = 0.5 in 2014), considering a 4 x 3 factorial scheme, complemented by Tukey test when effect of stimulants was significant.
The models adjusted were the first degree linear model for leaf number in both years; second degree for the other variables in 2013; and nonlinear model of Michaelis-Menten (Zeviani, 2013) in 2014: y = b1/(1+(x/b2)^b3), where y = response variable; x = day of the year (ordinal); b1 = maximum value of y; b2 = number of days needed for reducing 50 % the maximum y observed; b3 = parameter controlling the function form.
Results and Discussion
Experiment 1
The analysis of variance for the percentage of emergence revealed a significant effect of stimulants, on the contrary of seeding dates and the interaction seeding date x stimulants. The means for treatments with GA were significantly inferior to the others. ST and control did not differ from each other (Table 1). The percentage of emergence varied from 52.5 % to 64 % in the seeds treated with GA and in the other treatments, it was more than 71.3 %.
Table 1: Percentage of emergence and median time to emergence (T50, median and confidence interval - C.I.) of the passion fruit seeds under different seeding dates and stimulant treatments, in a greenhouse and subtropical climate. Chapecó, SC, Brazil, 2013.
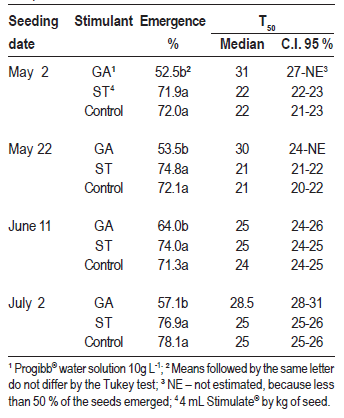
The median time for emergence (50 % of the plantlets, T50) was higher with the stimulant GA (Table 1), except when seeding occurred on June 11, when the confidence intervals were coincident. The effect of the treatments in the time needed for emergence can be visualized in Figure 1, derived from the survival analysis, where the curve of the proportion of not emerged seeds in treatment GA was slower and remained higher than the others in all the dates. Also, it was estimated a proportional emergence risk equal to 0.65 (p < 0.05) for the seeds treated with GA in relation to the control, what means GA acted against the emergence.
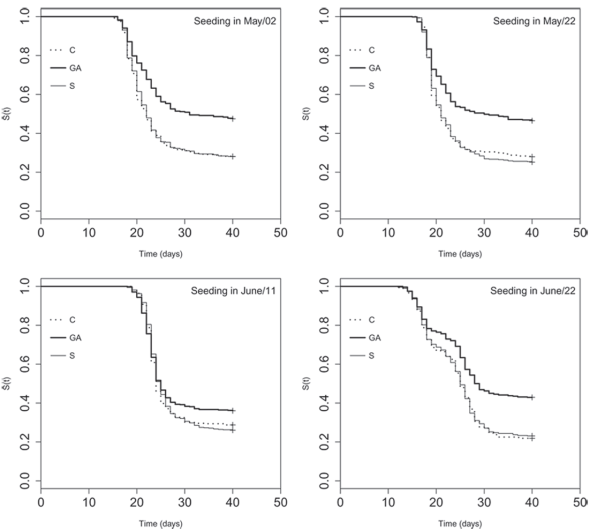
Figure 1: Estimate for Kaplan-Meier curves of the survival functions S(t) describing the time for the emergence of yellow passion fruit under treatments composed by seeding dates and stimulants (GA = Progibb® water solution 10 g L-1; S = 4 mL of Stimulate® by kg of seed; C = control).
The treatment Stimulate did not affect the median time to emergence, nor the proportional emergence risk, which was 0.99 (p > 0.05). It is possible that the higher T50 observed with the application of GA had been related to the reduction in the number of viable seeds. As a consequence, fewer seeds emerged along the whole period evaluated, delaying the attainment of 50 % of emergence.
The data presented revealed that immersing the seeds in GA 1.000 mg L-1 reduces the yellow passionfruit seeds emergence. This information contradicts the data published by Lima et al. (2009) who found an increase in germination until 1.000 mg L-1 and 96 h immersion. In another experiment in a greenhouse, doses up to 400 mg L-1 and immersion by 48 hours did not improve the germination (Cárdenas et al., 2013). Those conflicting results could be due to the interaction between doses and environmental conditions, as well as to the genotype studied (Souza et al., 2010; Malavasi, Dias & Malavasi, 2011).
The Stimulate (4 mL kg-1) did not affect the emergence. Ferraz et al. (2014) reported an increase in emergence with doses up to 30 mL kg-1 of P. edulis seeds. However, for the supposed increase no statistical confidence level was presented. On the other hand, Benjamin (2009), soaking the seeds in water solutions with increasing concentrations from 0 to 32 mL L-1 of Stimulate, observed significant effects, but the difference from the control was not supported by any statistical test. The difficulty in stablishing a pattern of response can be due to the compound formulation, which is a mix of three regulators, whose interaction promotes diverse effects when the dose or concentration is changed. Still, Ferreira et al. (2007) verified a higher percentage of early emergence with 12 and 16 mL kg-1, but 35 days after seeding the differences disappeared, which is in agreement with this work observations.
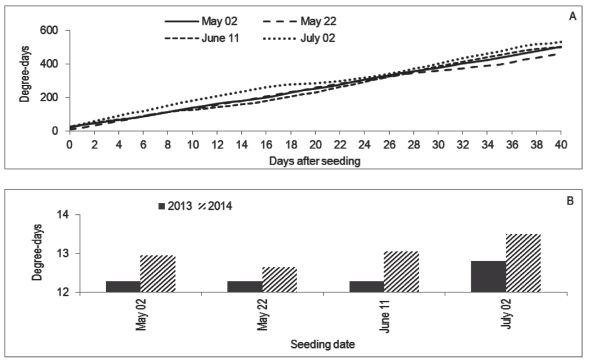
Seeding dates influenced all the variables. In the case of treatments with Stimulate and the control, T50 was smaller for the first two dates than for the two last ones, and the differences inside those pairs were not significant, given the overlapping of confidence intervals (Table 1). The difference occurred besides the similar amount of degree-days accumulated in the first three dates (Figure 2A). It is possible that temperatures inside the substrate had been different to the air, causing this discordance between degree-days and T50. In the comparison of seeding dates inside treatment GA, the upper limit of the confidence intervals could not be estimated, and so some comparisons are imprecise. However, it is possible that on June 11 the T50 had been smaller than on May 2 and July 2.
Experiments 2 and 3
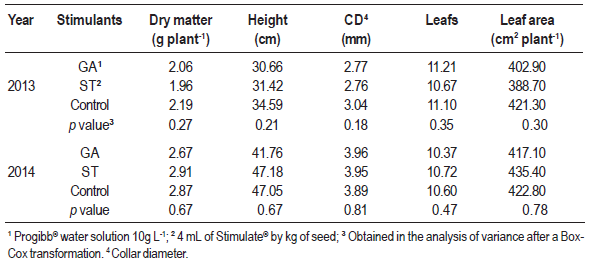
The application of GA and Stimulate in the seeds did not cause any alteration in dry matter, height, collar diameter, leaf number or leaf area of yellow passionfruit (Table 2). The interaction between seeding dates and stimulants was neither significant. However, seeding dates alone caused a significant effect.
Table 2: Characteristics of yellow passion fruit seedlings evaluated on September 15, following the application of Progibb® (GA) and Stimulate® (ST) and in a control, in 2013 and 2014, in Chapecó, SC, Brazil. Means of four seeding dates
The seedlings seeded in May 2 (121º day of the year) had mean height of 83 and 87.2 cm in 2013 and 2014, respectively (Figure 3), which is smaller than the observed by Zaccheo et al. (2013) in 120-days old seedlings seeded on May 15 in Londrina, PR, Brazil. In that place, the climate is Cfa, what suggests the environment in Chapecó was limiting to the growth, as can be observed in Figure 4: some days had an accumulation of less than 10 degree-days.
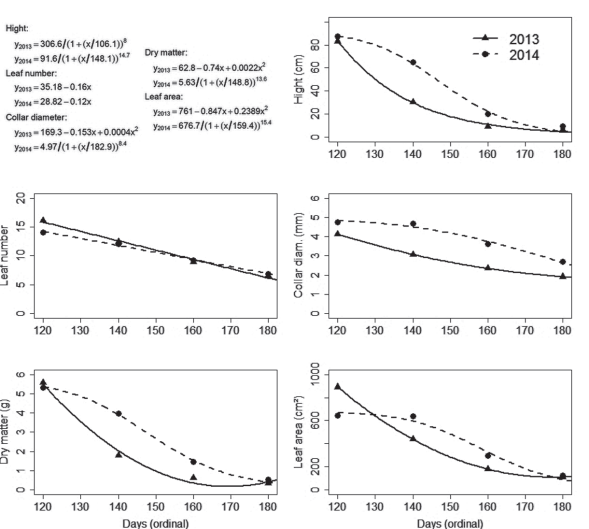
Figure 3: Models fitted to characteristics of yellow passion fruit seedlings, measured on September 15, 2013 and 2014, following the seeding dates May 2, May 22, June 11 and July 2 (121º, 141º, 161º and 182º day of the year, respectively), in Chapecó, SC, Brazil.
Shoot growth in the seedlings was not affected by the stimulants, even though they are considered stimulants of initial growth (Echer et al., 2006; Santos, 2010). As three seeds were seeded in each bag and plantlets were further thinned, the selection of the biggest plantlet could have annulled the effect of the treatments. This explanation fits the effect of the GA, which delayed and decreased the emergence rates without influencing the final plant growth.
The simple effect of seeding date on seedlings growth was significant, observed in height, leaf number and area, collar diameter and dry matter, for both years (Figure 3). However, differences in the pattern of response happened among characteristics in the same year and reciprocally, too.
For plant height, collar diameter, dry matter and leaf area, in 2014, the responses followed the Michaelis-Menten model (Zeviani, 2013). The higher value of y (response variable) was observed at the first seeding date, followed by a fall, which was more intense between the second and third dates, and tending to stability at the end of the period (Figure 3). The same variable responded as a linear quadratic function in 2013 when the fall in y was more intense from the first to the second dates. Leaf number, on the other hand, responded as a simple linear first-degree function, in both years. The number of leaves was reduced by 0.16 and 0.12 each oneday delay in seeding, in 2013 and 2014, respectively. The differences in the pattern of response between leaf number and the other variables are due to the increase in the intermodal space from the base to the apex of the shoot, i.e., small seedlings have a bigger number of leaves per centimeter of the shoot because the internodes are shorter.
The differences between years can be assigned to the different conditions of the environment. In Figure 4, it can be seen that for the same seeding date there were differences between years in the accumulation of degree-days in the days immediately subsequent to the seeding. In the cases with lower temperatures after the seeding (as after May 22, 2014) the initial daily growth rate is depressed, but it can be compensated by higher temperatures in the days ahead, along the growth period, resulting in similar sizes in the end of the experiment.
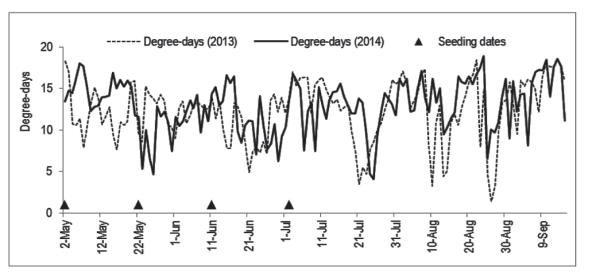
Figure 4: Daily accumulated degree-days (TB = 35 ºC; Tb = 15 ºC) in the greenhouse where yellow passion fruit seedlings from the Experiments 1, 2 and 3 were grown.
The effect of the seeding dates in the growth of the seedlings is due mainly to the differences in time (age of the plants in the end), but also to the differences in degree-days accumulation. As an example, seedlings seeded on June 11 and July 2, in 2014, suffered influences of a period of higher temperatures and accumulation of degree-days (Figure 2B) in comparison to the further seeding dates.
It is concluded that yellow passionfruit can be seeded anytime from May 2 to July 2 without losses in emergence percentage, which is about 70 %, even without a treatment with plant growth regulators. However, the earlier the seeding, the higher will be the seedlings to be transplanted in the field. Big-size seedlings generate more productive and precocious orchards in the first crop (Laredo, 2013). There is no biological advantage in using the stimulants tested when growing yellow passionfruit seedlings during subtropical autumn and winter of western Santa Catarina State; seeding from May 2 to 22 allows obtaining seedlings averaging 30 to 87 cm in height in mid of September.












 Curriculum ScienTI
Curriculum ScienTI