Services on Demand
Journal
Article
Related links
Share
Agrociencia (Uruguay)
Print version ISSN 1510-0839On-line version ISSN 2301-1548
Agrociencia Uruguay vol.20 no.2 Montevideo Dec. 2016
Synthesis and Field Evaluation of the Sex Pheromone of Plutella xylostella (L.) (Lepidoptera: Plutellidae) in Canola (Brassica napus L.)
Tacain Jimena1, Parpal Florencia1, Abbate Silvana2, Silva Horacio2, Ribeiro Adela2, Heguaburu Viviana1
1 Universidad de la República, Centro Universitario Regional Litoral Norte, Departamento de Química del Litoral.
2 Universidad de la República, Facultad de Agronomía, Departamento de Protección Vegetal. Avenida Garzón 780, 12900 Montevideo, Uruguay. Correo electrónico: vheguab@fq.edu.uy
Recibido: 9/6/15 Aceptado: 22/6/16
Summary
The diamondback moth Plutella xylostella (L.) is known to cause economic damage to rapeseed, cabbage, and other cruciferous crops worldwide. Sex pheromone components of P. xylostella, including (Z)-11-hexadecenal, (Z)-11-hexadecenol, and (Z)-11-hexadecenyl acetate, were synthesized in a concise and divergent fashion in yields appropriate to develop field tests. Two blends were prepared from these three components (8:18:100 and 10:1:90), and they were used to evaluate the number of P. xylostella adult male captures in commercial canola fields. Our results indicate that both blends were effective at attracting the microlepidoptera. Furthermore, they show that this type of pest-specific method could lead to the development of sustainable management strategies to rationalize the use of pesticides.
Keywords: CANOLA, PLUTELLA XYLOSTELLA, SEX PHEROMONE, SYNTHESIS, (Z)-11-HEXADECENAL
Sintesis y evaluación a campo de la feromona sexual de Plutella xylostella (L.) (Lepidóptera: Plutellidae) en canola (Brassica napus L.)
Resumen
La polilla dorso de diamante Plutella xylostella (L.) causa daños económicos en canola y otras crucíferas. Los componentes de la feromona sexual de P. xylostella, (Z)-11-hexadecenal, (Z) -11-hexadecenol y acetato de (Z)-11-hexadecenilo, fueron sintetizados por medio de una estrategia concisa y divergente, obteniéndose cantidades adecuadas para el desarrollo de ensayos de campo. Dos formulaciones fueron preparadas a partir de los tres componentes (8:18:100 e 10:1:90) para evaluar el número de machos adultos de P. xylostella capturados en un campo comercial de canola. Nuestros resultados indican que ambas formulaciones fueron eficaces en atraer al microlepidóptero. Los resultados obtenidos son promisorios y muestran que este método específico para plagas permite el desarrollo de estrategias de manejo sustentable para racionalizar el uso de pesticidas.
Palabras clave: CANOLA, PLUTELLA XYLOSTELLA, FEROMONA SEXUAL, SÍNTESIS, (Z)-11-HEXADECENAL
Introduction
Canola (Brassica napus L.) is a cruciferous oleaginous with high production levels worldwide, being the second most important oleaginous crop after soybean (FAO, 2013). Due to its ability to grow and develop in low temperatures, it is one of the few oleaginous adaptable to wide extensions. It is characterized for possessing oil with excellent quality for human consumption and for the preparation of biofuel, as well as an extraction residue with high protein level for animal feed. In Uruguay, canola appeared as a promising alternative for crop rotation instead of the usual winter cereals. As a matter of fact, this practice has been promoted by Alcoholes del Uruguay (ALUR) for biodiesel, glycerin, and protein wheat production in an effort to achieve high levels of bio-renewable energy (Uruguay. Poder Legislativo, 2007). These types of policies allowed Uruguay to develop an energy matrix in which biomass is represented by 30 %, in line with the Kyoto protocol, by contributing with the sustainable development of the region.
The diamondback moth, Plutella xylostella (L.) (Lepidoptera: Plutellidae) is one of the most destructive insects of cruciferous plants throughout the world (Talekar and Shelton, 1993), being the major pest of canola in South America and other regions. This microlepidoptera has a wide distribution due to its ability to adapt to a broad range of weathers and to cover large expanses through migrations (Nguyen et al., 2014). The lack of effective natural enemies is considered to be the major cause of the high pest status in most parts of the world (Lim, 1986). During bloom, and until the start of the development of pods, canola field monitoring must be performed in order to check for P. xylostella larvae. Early monitoring of adults and larvae, and the rational use of insecticides when populations are above threshold levels, is crucial for pest management and to avoid economic loss by diamondback moth damage. Chemical control in canola is particularly challenging, as insecticides are required in the fructification stage and this coincides with the presence of beneficial insects and pollinators which frequent the crop. For this reason, the choice of selective insecticides is essential. Moreover, the effectiveness of insecticides has been reduced due to the development of resistance to many of these compounds (Lee et al., 1993; Chung et al., 1997). P. xylostella is known for having resistance to pyrethroids (Liu et al., 1982), organophosphorus (Miyata et al., 1982), growth regulators (Lin et al., 1989), and Bacilus thuringensis (Hama et al., 1992), among others. Indeed, the diamondback moth was the first insect found to have become resistant to B. thuringensis toxin in the field (Tabashnik et al., 1997; Safraz et al., 2005; Grzywacz et al., 2010). Therefore, alternative control strategies to the sustained use of synthetic insecticides are urgently needed.
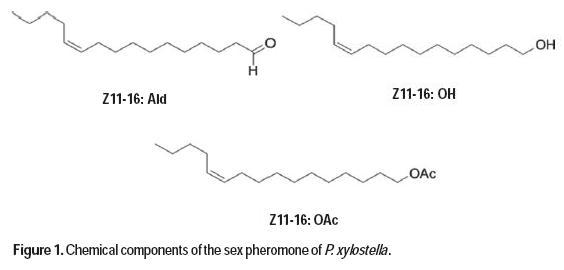
With the use of sex pheromones, the population of insect pests can be reduced, the presence of insects of agricultural interest may be detected, the behavior of pest populations can be learned, and decisions can be made regarding the adequate use of insecticides (Zarbin et al., 2007). The P. xylostella sex pheromone can be used for monitoring as well as for mating disruption of this species (Yang et al., 2007). Pheromone traps have been used to monitor adult populations, and can predict the next generation of larval population peaks occurring 11 to 21 after peak adult trap catch (Miluch et al., 2013). The sex pheromone of P. xylostella was first isolated from female abdominal preparations (Chow et al., 1974), and later identified (Z)-11-hexadecenal (Z11-16:Ald) and (Z)-11-hexadecenyl acetate (Z11-16:Ac) as the components in the female pheromone gland extract (Tamaki et al., 1977). The addition of (Z)-11-hexadecenol (Z11-16:OH) further synergized male response to the first two components (Koshihara and Yamada, 1980), and it was later shown to be a minor pheromone component (Suckling et al., 2002). Analyses of the female gland extract (Yang et al., 2007) demonstrated that the ratio of Z11-16:Ald, Z11-16:OH, and Z11-16:Ac found was 8:18:100, respectively. However, significant geographical variations in sex pheromone production and response have been reported in P. xylostella (Suckling et al., 2002; Chisholm et al., 1979). Different research groups have used different ratios of the main pheromone components during tests or in pheromone monitoring. In South Korea (Yang et al., 2007), researchers found that the ternary blend of Z11-16:Ald, Z11-16:OH, and Z11-16:Ac at a ratio 10:1:90 was more effective at caching P. xylostella males than the natural ratio and other reported blends (Figure 1).
Due to the worldwide distribution and pest status of the diamondback moth, populations are monitored in brassicaceous cropping systems around the world. Synthetic sex pheromone lures to monitor adult diamondback moth populations are available from many different companies, but the efficacy of these lures is variable (He et al., 2003), and the information regarding the comparison between different available lures in one geographic location is scarce (Evenden and Gries, 2010). The promotion of canola crops in Uruguay for biodiesel production led us to compare the attractiveness of different synthetic pheromone blends to diamondback moth males for their potential use as a monitoring system. In this report we present a concise and divergent synthesis of the three pheromone components, as well as field tests with two different blends, to evaluate the number of P. xylostella adult male captures in commercial canola fields.
Materials and Methods
Synthesis
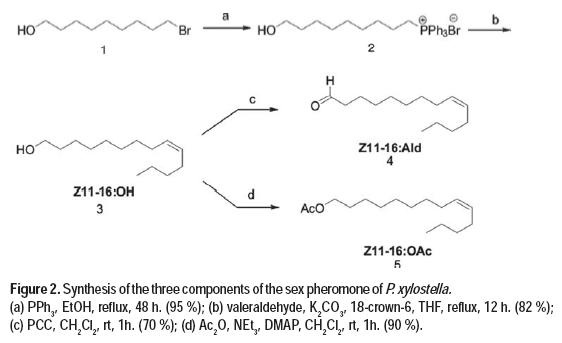
The three components of the sex pheromone of P. xylostella, (Z)-11-hexadecenol, (Z)-11-hexadecenal and (Z)-11-hexadecenyl acetate (Chow et al., 1974; Suckling et al., 2002), were prepared according to the below synthetic protocol, in order to perform field experiments.
Preparation of 11-hydroxyundecyltriphenylphosphonium bromide (2): 11-bromo-1-undecanol (1, 24.7 g, 98.3 mmol) and triphenylphosphine (38.6 g, 147.5 mmol) were dissolved in absolute etanol (100mL), and the mixture was heated to reflux for 48 h. Crystallization by addition of hexanes and wash of the solid obtained with hexanes gave pure 11-hydroxyundeciltriphenylphosphonium bromide in 95 % yield.
Preparation of (Z)-11-hexadecenol (3): 11-Hydroxyundeciltriphenylphosphonium bromide (2) (1.5 g, 2.9 mmol) and solid potassium carbonate (0.4 g, 2.9 mmol) were dissolved in the minimum amount of tetrahydrofuran, and a tip of spatula of 18:crown:6 ether was added at 0 ºC. Pentanal (0.2 mL, 2.7 mmol) was added and the reaction mixture was heated to reflux for 12 hours. The solvent was distilled under vacuum and the residue purified by column chromatography on silica gel using hexane: ethyl acetate (1:1) mixture, to obtain (Z)-11-hexadecenol in 82 % yield.
Preparation of (Z)-11-hexadecenal (4): (Z)-11-hexadecenol (3) (0.78 g, 3.24 mmol) and pyridinium chlorochromate (PCC) (1.04 g, 4.86 mmol) were stirred in CH2Cl2 (30 mL) at room temperature for 1 hour. The reaction mixture was then filtered through celite, the solvent evaporated, and the residue purified by column chromatography on silica gel using hexane: ethyl acetate (9:1) mixture, affording 4 in 70 % yield.
Preparation of (Z)-11-hexadecenyl acetate (5): (Z)-11-hexadecenol (3) (2.65 g, 11 mmol) was dissolved in dry dichloromethane under a nitrogen atmosphere. Triethylamine (9.2 mL, 66 mmol), acetic anhydride (3.1 mL, 33 mmol) and a spatula tip of 4-dimethyaminopyridine were added while stirring the solution in an ice bath. Upon finished the reaction (1 hour), the crude was washed with a cold aqueous saturated solution of sodium carbonate and with 5 % hydrochloric acid aqueous solution. The product was purified by column chromatography using a hexane: ethyl acetate (9:1) mixture, affording 5 in a 90 % yield.
Once these tree compounds ((Z)-11-hexadecenol, (Z)-11-hexadecenal and (Z)-11-hexadecenyl acetate) were synthesized, two blends were prepared with different composition, for their use as treatments in field experiments.
Field Experiments
Pheromone blends were evaluated in white rubber septa (Sigma-Aldrich, white, 8 mm O.D.) placed in the center of handcrafted plastic Delta traps (15 × 15 × 25 cm). The traps were distributed within a commercial canola field (56 ha) situated in Paysandú, Uruguay (32.5 ° South, 57.8 ° West), planted with the Rivette variety in furrows. No insecticides were applied throughout the crop cycle. The experiment was conducted for seven weeks during spring time. Larvae density before installing the traps was estimated by individual plant examination (Dosdall et al., 2011). The treatments were blends of the synthetic pheromone (Z11-16:Ald, Z11-16:OH, Z11-16:Ac) in 8:18:100 and 10:1:90 composition, with a total septum load of 0.1 mg in 100 µL of hexane. The choice of the septa load was made in agreement with previous reports (Miluch et al., 2014; Yang et al., 2007). Septa loaded with hexane were used as controls. The experiment was arranged in a randomized block design, with seven blocks separated by 60 m, and 30 m trap separation within a block. The traps were hanged just above the height of the foliage. A global positioning system was used for the location of traps. Male captures were checked weekly. The sticky bases of the traps were changed as needed, according to the number of males captured. From the fifth week, new treatments were added to the seven blocks in order to check the effect of volatility or decomposition in the sex pheromone components in field conditions.
Statistical Analysis
For each capture date, a generalized mixed model was fitted. The number of captured males was the response variable, the treatment was considered as a fixed factor and the block as a random factor. Data were analyzed using package «Ime4» and provided in the R statistical software. Means between treatments were compared using «multcomp» package and considered significant if p < 0.05 in Tukey´s test.
Results and Discussion
The three components of P. xylostella sex pheromone are (Z)-unsaturated long chain related compounds. The general synthetic scheme involved a stereoselective Wittig reaction performed in Boden conditions, and further oxidation or acylation. In this way, the synthesis of (Z)-11-hexadecenol started from commercially available 11-bromoundecanol (1), with the formation of the corresponding phosphonium salt (2). This was followed by Wittig reaction between 2 and valeraldehyde, which yielded the sex pheromone component (Z)-11-hexadecenol in 82 % combined yield for the two steps. The (Z) isomer is here obtained with high stereoselectivity, with a (Z)/(E) ratio of 9/1. The final stage of the synthetic sequence is the oxidation of (Z)-11-hexadecenol to the pheromone component (Z)-11-hexadecenal (Sellanes et al., 2010) using pyridinium chlorochromate as oxidizing agent in 70 % yield. Acetylation of (Z)-11-hexadecenol with acetic anhydride in the presence of triethylamine and 4-dimethylaminopyridine afforded (Z)-11-hexadecenyl acetate in excellent yield (90 %) (Figure 2).
The pheromone components were prepared through a concise synthetic design based on a Wittig olefination, and further functionalization of the oxygenated moiety. This strategy avoids the use of protecting groups and does not afford complex mixtures of stereoisomers. Overall, the desired pheromone components were prepared in a stereoselective fashion, using a concise divergent three step synthesis with overall yields ranging from 54 to 78 % for each component, therefore representing a more efficient approach than those previously reported (Szantay et al., 1981; Zong et al., 2011; Xun et al., 1985).
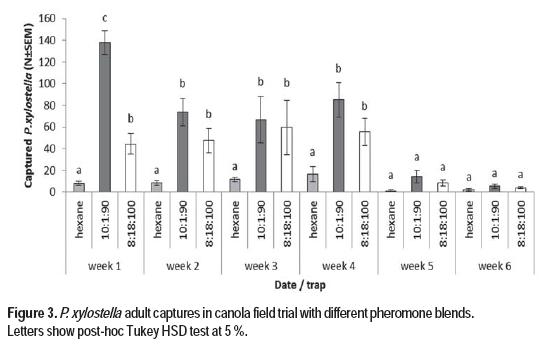
Field test were performed in an infested commercial canola field, and the treatments were two blends of the synthetic pheromone (Z11-16:Ald, Z11-16:OH, Z11-16:Ac) in 8:18:100 and 10:1:90 ratio (Figure 3).
Field experiments were carried out at the blooming stage when the population of P. xylostella was settled and there was an average of 3,2 larvae per plant. This value is higher than the action threshold for chemical control in canola (Western Committee on Crop Pests, 2010). At the initial sampling time, one week old lures baited with synthetic pheromone in the two tested ratios (Z11-16:Ald, Z11-16:OH, Z11-16:Ac), catched a significantly higher number of moths than the control traps baited with hexane (Tukey’s test, p < 0.05). At this point, the 10:1:90 blend was more attractive than the 8:18:100 blend (Tukey’s test, p< 0.05). From the second to the fourth week, both pheromone treatments captured more moths than the control, but there were no differences between them (Tukey’s test, p < 0.05). The efficiency of the 10:1:90 blend has been previously reported with cabbages (Yang et al., 2007). This finding is in line with a previous report (Evenden and Gries, 2010), that studied the release rate of these pheromone components in field tests, showing that there is an initial burst of pheromone release that is diminished over the time under field conditions. Both blends have Z11-16:Ac as the main component, in line with the observation of Miluch et al. (2014), that points that this main component attracts significantly more moths than those in which Z11-16:Ald is the main component. The 8:18:100 blend, which mimicked the ratio found in female gland extracts, has Z11-16:OH as it second major component, while the 10:1.90 blend has Z11-16:Ald as it second major component. As reported earlier (Môttus et al., 1997), the aldehyde component (Z11-16:Ald) is volatilized and decomposed faster than the other components. Therefore, after the second week in field conditions, the blend composition may be altered approaching a similar ratio between the two treatments, showing similar efficacy. Differences between hexane and pheromone treatments were detected until the fourth week of sampling when none of the traps captured a significant number of male moths. This is in agreement with the end of the last generation of P. xylostella at the ripening stage of the crop. In order to confirm that this last observation was not due to the loss of pheromone components in field conditions, new treatments were added to the seven blocks at the fifth week. This strategy was chosen over septa replacement because several reports pointed that the traps baited with lures aged under field conditions showed better results in comparison to traps baited with fresh lures (Miluch et al., 2013; Evenden and Gries, 2010). No significant differences were observed between these new set of treatments and those installed earlier (8:18:100 blend: z=-1,77, p=0,39; 10:1:90 blend: z=-1,23 p=0,72).
Monitoring of diamondback moth in canola throughout the crop cycle is usually conducted using sweep net sampling or individual plant examinations. The first method does not provide an idea of larvae density, therefore the most accurate method of estimating this pest population density in canola is to perform counts of diamondback moth specimens per plant (Leoncelli et al., 2013; Miluch et al., 2013). Finding larvae on each plant is a difficult and slow process which involves a high sample effort, especially at the first stage of the larvae when mine within the leaf. Selective chemical intervention is effective until the third larval instar of the pest and pheromone traps are a valuable tool to monitor their development. Miluch et al. (2013) determined that when P. xylostella populations are established, moth capture from one pheromone-baited trap per field can predict larval populations, and this information can be used to determine the optimal initial sampling date.
Considering that females of P. xylostella usually mate and oviposit in the first 24 h of life (Talekar and Shelton, 1993), the first capture in pheromone-baited trap can be used as the date to begin rate summation to apply the stochastic model proposed by Marchioro et al. (2016). This model accurately estimates the larval emergence of P. xylostella under field conditions in southern Brazil and could offer a promising tool for Integrated Pest Management purposes, although it has to be tested in canola at these latitudes. Moth captures by pheromone traps will allow the implementation of sequential sampling for larval in canola using the model proposed in Argentina by Lietti et al. (2014).
Our results suggest than both blends placed on Delta sticky traps have the potential to be developed for commercial use to monitor diamondback moth population. Future research should determine the optimum timing of insecticide applications using threshold captures in sex pheromone traps, as it was determined for P. xylostella in cabbage (Brassica oleracea var capitata) and cauliflower (B. oleracea var botrytis) (Reddy and Guerrero, 2001).
Conclusions
The three components of the sex pheromone of P. xylostella were synthesized through an efficient methodology that allows for future scale-up. The two blends employed in canola field tests were effective. The use of a specific monitoring method for this pest will allow choosing the proper time to use a selective insecticide (i.e. chitin synthesis inhibitor) and the development of a sustainable management strategy in a crop which, as seen by its explosive growth, has gained popularity among producers. Further work is needed in order to develop a monitoring strategy for massive use. Parameters such as septum load and duration, trap height and color, as well as threshold capture levels, need to be optimized for a final monitoring technology (Môttus et al., 1997; Miluch et al., 2013, 2014). It was shown that the two key factors are possible to achieve: a convenient and scalable synthetic route and the capture of males in field traps baited with synthetic pheromone. Field tests performed in this region have an added-value, since significant geographical variations in sex pheromone production and response have been reported for this species (Miluch et al., 2014; Yang et al., 2007). Therefore, population monitoring of P. xylostella in canola crops is practical and feasible, and may result in a significant reduction in insecticide use when chemical control is employed preventively.
References
Chisholm MD, Underhill EW, Steck WF. 1979. Field trapping of the diamondback moth (Lepidoptera: Plutellidae) using synthetic sex attractants. Environmental Entomology, 8: 516 – 518.
Chow YS, Chiu SC, Chien CC. 1974. Demonstration of sex pheromone of the diamondback moth (Lepidoptera: Plutellidae). Annals of the Entomological Society of America, 67: 510 – 512.
Chung BK, Kang SW, Choo HY. 1997. Joint toxic action of bifenthrin and prothiofos mixture for the control of insecticide-resistant diamondback moth, Plutella xylostella L. Korean Journal of Applied Entomology, 36: 105 – 110.
Dosdall L, Soroka J, Olfert O. 2011. The diamondback moth in canola and mustard: current pest status and future prospects. Prairie Soils and Crops, 4: 66 – 76.
Evenden ML, Gries R. 2010. Assessment of commercially available pheromone lures for monitoring diamondback moth (Lepidoptera: Plutellidae) in canola. Journal of Economic Entomology, 103(3): 654 – 661.
FAO. 2013. Statistical Yearbook [On line]. Cited April 2016. Available from: http://faostat.fao.org/site/291/default.aspx.
Grzywacz D, Rossbach A, Rauf A, Russel DA, Srinivasan R, Shelton AM. 2010. Current control methods for diamondback moth and other brassica insect pests and the prospects for improved management with lepidopteran-resistant Bt vegetable brassicas in Asia and Africa. Crop Protection, 29: 68 – 79.
Hama H, Suzuki K, Tanaka H. 1992. Inheritance and stability of resistance to Bacilus thuringiensis formulations of the diamondback moth, Plutella xylostella (Linnaeus) (Lepidoptera: Yponomeutidae). Applied Entomology and Zoology, 27: 355 – 362.
He X, Chen W, Liu TX. 2003. Attraction of diamondback moth to three commercial sex pheromone lures under laboratory and field conditions. Southwestern Entomologist, 28: 105 – 114.
Koshihara T, Yamada H. 1980. Attractant activity of female sex pheromone of diamondback moth Plutella xylostella (L.). Japan Journal of Applied Entomology and Zoology, 24: 6 – 12.
Lee SC, Cho YS, Kim DI. 1993. Comparative study of toxicological methods and field resistant in diamondback moth (Lepidoptera: Plutellidae). Korean Journal of Applied Entomology, 32: 323 – 329.
Leoncelli G, Fernández C, Lietti M. 2013. Monitoreo de las polilla de las coles, Plutella xylostella (L.), con trampa de feromona en colza. In: Encuentro de Jóvenes Investigadores de la Universidad Nacional de Litoral XVII; 4 - 5 setiembre; 2013; Santa Fe, Argentina. Santa Fe : Universidad Nacional del Litoral. p. 4.
Lietti M, Trumper E, Fernández C, Reyes V, Leoncelli G, Vignaroli L. 2014. Plan de muestreo secuencial para larvas de la polilla de las coles Plutella xylostella (L.), en colza. In: 1er Simposio Latino Americano de Canola; 19 - 21 agosto; 2014; Passo Fundo, RS. Brasil. Brasilia : Embrapa. pp. 11- 17.
Lim GS. 1986. Biological Control of Diamondback Moth. In: Proceedings of the First International Workshop, Asian Vegetable Research and Development Center. Shanhua, Taiwan : Talekar NS and Griggs TD. pp. 159 – 171.
Lin JG, Hung CH, Siin CN. 1989. Teflubenzuron resistance and microsomal monooxygenases in larvae of the diamondback moth. Pesticide Biochemistry Physiology, 35: 20 – 25.
Liu MY, Tzeng YJ, Sun CN. 1982. Diamondback moth resistance to several synthetic phyretroids. Journal of Economic Entomology, 74: 393 – 396.
Marchioro CA, Krechemer FS, Moraes CP, Foerster LA. 2016. A stochastic model for predicting the stage emergence of Plutella xylostella under field conditions. Annals of Applied Biology, 169: 190 – 199.
Miluch C, Dosdall LM, Evenden ML. 2014. Factors influencing male Plutella xylostella (Lepidoptera: Plutellidae) capture rates in sex pheromone-baited traps on canola in western canada. Journal of Economic Entomology, 107(6): 2067 – 2076.
Miluch C, Dosdall LM, Evenden ML. 2013. The potential for pheromone-based monitoring to predict larval populations of diamondback moth, Plutella xylostella (L.), in canola (Brassica napus L.). Crop Protection, 45: 89 – 97.
Miyata T, Kawai H, Saito T. 1982. Insecticide resistance in the diamondback moth Plutella xylostella L. (Lepidoptera: Yponomeutidae). Applied Entomology and Zoology, 17: 539 – 542.
Môttus E, Nôm V, Williams H, Liblikas I. 1997. Optimization of pheromone dispensers for diamondback moth Plutella xylostella. Journal of Chemical Ecology, 23(9): 2145 – 2159.
Nguyen C, Bahar MH, Baker G, Andrew NR. 2014. Thermal tolerance limits of diamondback moth in ramping and plunging assays. PLOS ONE, 9(1): e87535.
Reddy GVP, Guerrero A. 2001. Optimum timing of insecticide applications against diamondback moth Plutella xylostella in cole crops using threshold catches in sex pheromone traps. Pest Management Science, 57: 90 – 94.
Safraz M, Keddie AB, Dosdall LM. 2005. Biological control of the diamondback moth, Plutella xylostella : A review. Biocontrol Science and Technology, 15: 763 – 789.
Sellanes C, Rossini C, González A. 2010. Formate analogs as antagonists of the sex pheromone of the honeydew moth, Cryptoblabes gnidiella: Electrophysiological, behavioral and field evidence. Journal of Chemical Ecology, 36: 1234 – 1240.
Suckling DM, Gibb AR, Daly JM, Rogers DJ, Walker GP. 2002. Improving the pheromone lure for diamondback moth. New Zealand Plant Protection, 55: 182 – 187.
Szantay C, Novák L, Toth M, Stefko B, Kis-Tamas A, inventors; Egyt Gyogyszervegyeszeti Gyar, assignee. 1981 Jan 6. Composite insect attractant for male cabbage moths and a process for preparing its active agents. United State patent US 4,243,660.
Tabashnik BE, Liu Y, Finson N, Masson L, Heckel DG. 1997. One gene in diamondback moth confers resistance to four Bacillus thuringiensis toxins. Proceedings of the National Academy of Sciences of the USA, 94: 1640 – 1644.
Talekar NS, Shelton AM. 1993. Biology, ecology, and management of the diamondback moth. Annual Review of Entomology, 38: 275 – 301.
Tamaki Y, Kawasaki K, Yamada H, Koshihara T, Osaki N, Ando T, Yoshida S, Kankiohana H. 1977. (Z)-11-Hexadecenal and (Z)-11 hexadecenyl acetate : Sex pheromone components of the diamondback moth (Lepidoptera: Plutellidae). Applied Entomology and Zoology, 12: 208 – 210.
Uruguay. Poder Legislativo. 2007. Ley Nº 18.195. Agrocombustibles : Se regula su fomento y regularización de su producción, comercialización y utilización. 14/11/2007.
Western Committee on Crop Pests. 2010. Guidelines for the control of insect pests [On line]. Cited April 2016. Available from: http://www.westernforum.org/Documents/WCCP/WCCP_documents/WCCP_Guidelines/WCCP_16/Oilseeds-WCCP-2016.pdf.
Xun L, Zhang Z, Jie K, Pickett JA, Yongcheng P, Yige X, Jiechen G. 1985. Field attractant activity of the synthetic sex pheromone of diamondback moth, Plutella xylostella. Acta Ecologica Sinica, 5(3): 249 – 256.
Yang CY, Lee S, Choi KS, Jeon HY, Boo KS. 2007. Sex pheromone production and response in korean populations of the diamondback moth, Plutella xylostella. Entomologia Experimentalis et Applicata, 124: 293 – 298.
Zarbin PHG, Villar JAFP, Corrêa AG. 2007. Insect pheromone synthesis in Brazil: an overview. Journal of the Brazilian Chemical Society, 18(6): 1100 – 1124.
Zong GH, Yan SQ, Liang XM, Wang DQ, Zhang JJ. 2011. Synthesis of the sex pheromone of P. xylostella (L.). Chinese Journal of Organic Chemistry, 31(12): 2126 - 2130.