Services on Demand
Journal
Article
Related links
Share
Agrociencia (Uruguay)
Print version ISSN 1510-0839On-line version ISSN 2301-1548
Agrociencia Uruguay vol.19 no.2 Montevideo Dec. 2015
Production of Lipase by Aspergillus fumigatus in Solid State Fermentation Using Residues from Rice
Castiglioni Gabriel Luis1, Costa Jorge Alberto Vieira2
1Escola de Agronomia,Universidade Federal de Goiás, Setor de Engenharia de Alimentos, Campus Samambaia. Rodovia Goiânia / Nova Veneza, km 0, Caixa Postal 131, CEP 74690-900, Goiânia GO, Brasil
E-mail: gabrielcastigli@gmail.com
2Escola de Química e Alimentos, Universidade Federal de Rio Grande, Campus Carreiros. Avenida Itália, km 08, Caixa Postal 474, 96.201-900 Rio Grande, RS, Brasil
Recibido: 17/6/14 Aceptado: 28/9/15
Summary
Many factors can influence the production of lipase in a fermentation process, among them the air supply and the type of substrate. Biotechnological advances in industrial production of lipase have been allowing the application of this enzyme in different sectors, making it economically viable. This study aimed to evaluate the production of lipase by Aspergillus fumigatus in different nutritional and aeration conditions. The experiments were performed in column bioreactors aerated with 0, 40, 60, 80, 120 and 200 mLair.gmedium-1.h-1, in a medium with no additional carbon source and containing soybean oil and diesel. Significant difference (p <0.05) was observed in the results of lipase activity. The best activities were 103.13, 105.76 and 100.19 U.g-1 using 80 mLair.gmedium-1.h-1 for 48, 72 and 48 h of fermentation, for experiments with no additional carbon source containing soybean oil and diesel, respectively.
Keywords: ASPERGILLUS FUMIGATUS, SOLID-STATE FERMENTATION, LIPASE
Resumen
Producción de lipasa por Aspergillus fumigatus en fermentación en estado sólido utilizando residuos de arroz
Muchos factores pueden influenciar el proceso de producción de las lipasas. Entre ellos se encuentran el suministro de aire y el tipo de sustrato. Avances biotecnológicos en la producción industrial de la lipasa han permitido la utilización de esta enzima en distintos sectores, siendo esto económicamente viable. Este trabajo tuvo como objetivo evaluar la producción de lipasa por Aspergillus fumigatus en distintas condiciones nutricionales y de aireación. Los experimentos se llevaron a cabo en biorreactores de columna con medio de cultivo sin fuente adicional de carbono, aceite de soja y diesel, bien como 0, 40, 60, 80, 120 y 200 mLaire.gmedio-1.h-1. Se observó diferencia significativa (p < 0,05) en los resultados de actividad enzimática. Las mejores actividades fueron 103,13, 105,76 y 100,19 U.g-1 usando 80 mLaire.gmedio-1.h-1, sin fuentes adicionales de carbono, añadiendo aceite de soja y diesel, respectivamente.
Palabras clave: ASPERGILLUS FUMIGATUS, FERMENTACIÓN EN ESTADO SÓLIDO, LIPASA
Introduction
Lipases (triacylglycerol hydrolase EC 3.1.1.3) catalyze reactions involving hydrolysis and synthesis of ester from glycerol and long chain fatty acids. Their biotechnological potential is due to the high stability in organic solvents, no requirement for cofactors, high substrate specificity and high enantioselectivity (Castro and Anderson, 1995). This characteristic also enables some lipases catalyze synthetic reactions (Carvalho et al., 2006).
Lipases have a great industrial importance such as in the synthesis of food ingredients (Somashekar and Divakar, 2007), used as detergent additives (Liu et al., 2009), in the synthesis of intermediates of medicines and other products of light industry (Wang et al., 2009). In addition to the industrial applicability, lipases can be used in the biodegradation of effluents from dairy, meat, vegetable oils, and other industries (Hiol et al., 2000).
Lipases are produced by a large number of microorganisms including bacteria, yeasts and molds. The preference for the industrial use of fungi is usually attributed to the extracellular production of lipase, which facilitates extraction of the fermented medium (Carvalho et al., 2005). The most suitable among the fungi are the genera Rhizopus sp., Mucor sp., Geotrichum sp., Penicillium sp. and Aspergillus sp. (Hita et al., 2009). It should be noted that among the different species, Aspergillus oryzae and niger are considered as safe microorganisms (GRAS) by the Food and Drug Administration (FDA) in the United States list. Martins et al. (2008), Naqvi et al. (2013) and Shangguan et al. (2012) show the potential of Aspergillus fumigatus for lipase production in solid state fermentation (SSF). The major advantages attributed to this type of fermentation refer to high rates of cell growth and low proteolytic activity (Diaz et al., 2006). Castilho et al. (2000) also mention that the results related to investments in SSF are significantly lower when compared with submerged fermentation.
The SSF is considered a suitable technology for enzyme production, since this process can be conducted in situ and use agro-industrial residues as a source of nutrients (Cammarota and Freire, 2006). Thus, the search for different carbon sources for lipase production becomes an alternative for minimizing production costs (Burkert et al., 2004; Ciafardini et al., 2006; You Li et al., 2004). Another significant factor in the production of many metabolites by fungi is the aeration of the fermentation medium (Hongzhang et al., 2002).
The objective of this study was to evaluate the production of lipase by Aspergillus fumigatus in solid state fermentation using different aeration conditions and additional carbon sources.
Materials and Methods
Microorganism
We used the strain of the filamentous fungus Aspergillus fumigatus provided by the Laboratory of Biochemical Engineering, at Universidade Federal do Rio Grande. The microorganism was maintained at 4 °C in test tubes with potato dextrose agar (PDA) and added 1 % glycerin.
Fermentation System
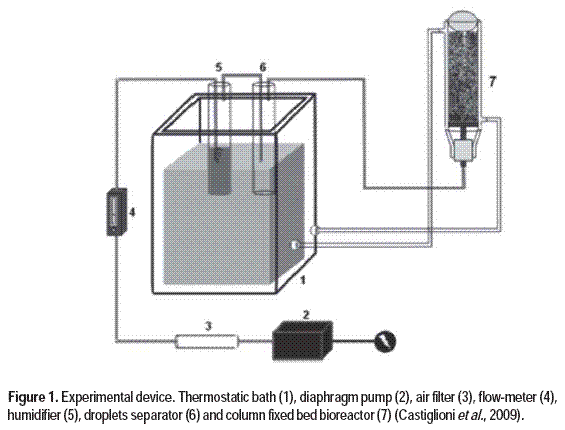
The culture media were conducted in the system described by Hasan et al. (1998). Fermentations were performed in packed-column bioreactors with internal dimensions of 50 mm diameter and 250 mm height. The air supplied by diaphragm pumps was filtered, humidified and transferred to the drop eliminator, in order to maintain constant air humidity. Air injection for each experiment was controlled by rotameters installed at the base of each bioreactor. The experiment is outlined in Figure 1.
Fermentation
The growth medium was composed of rice hulls and defatted rice bran from Impresa Riograndense de Óleo Vegetal - Irgovel. The bran was ground in a Wiley mill using a 1 mm mesh. After grinding, the bran was sieved and particles retained between sieves 35 and 32 in the Tyler series (0.42 and 0.50 mm, respectively) were collected. Besides the hull and rice bran (15:85), the fermentative medium also contained 90 mL of nutrient solution composed of (g.L-1): MgSO4.7H2O (0.5), NaNO3 (3.0), KH2PO4 (1.0), yeast extract (1.0) and peptone (0.3). The nutrients used in the experiments were of analytical standard from Synth.
Soybean or diesel oils were added in separate experiments at a ratio of 1 % (w/w), as an additional carbon source, for further comparison with experiments without their addition. Besides, cultures without air supply, aerations of 40, 60, 80, 120 and 200 mLair.gmedium-1.h-1 were evaluated, aiming to study a wide range of this variable.
The inoculum was prepared with nutrient agar in Roux bottles for 96 h, for maximum sporulation. After this period, spore scraping was performed with aqueous Tween 80 (1 %) and an initial concentration of 4.106 spores.gmedium-1 was added to the fermentation medium. Experimental conditions were 30 °C for 144 h, pH 4.5 and 50 % humidity.
Enzyme Extraction
For enzyme extraction, a 50 mM phosphate buffer (pH 7.0) 1:10 (w/v) was added. Then, the sample was stirred in shaker at 160 rpm and 30 °C for 30 min and filtered under vacuum in a Büchner funnel, obtaining the extract to evaluate the lipolytic activity.
Determination of Lipolytic Activity
The lipolytic activity was determined according to the method described by Macedo et al. (1997), using 2.5 mL of olive oil, 7.5 mL of 5 % aqueous solution of gum arabic, 5 mL of 10 mM phosphate buffer (pH 7.0) and enzyme extract in 1 mL emulsion. The mixture was incubated at 37 °C for 30 min under stirring in shaker at 150 rpm and the reaction solution was stopped with acetone:ethanol 1:1 v/v. Fatty acids released during the reaction of emulsion and enzymatic extract were titrated with NaOH 0.05 N. One unit of lipase activity was defined as the amount of enzyme which releases 1 ìmol of fatty acid per minute.
The moisture of the samples was determined according to the AOAC (1995) for subsequent conversion of the results to a dry basis. All analyzes were performed in triplicate.
Analysis of Results
The results of lipase activity found for the different cultivation conditions were evaluated by analysis of variance using univariate and multivariate R software, version 3.0.1 (Good Sport). In order to check the similarity among experiments, cluster analysis using the Manhattan method was performed, considering the distance among the closest results of lipase activity as the distance between the clusters.
Results and Discussion
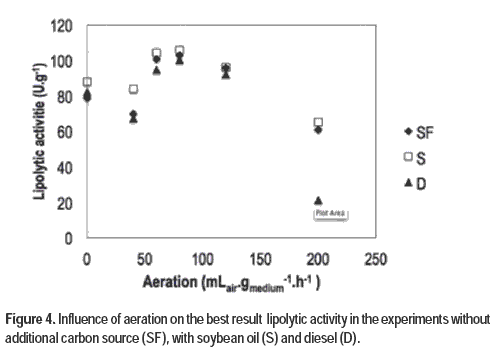
Figure 2 shows the evolution of the lipase production in different aeration conditions of the fermentation process by the fungus Aspergillus fumigatus.
The available oxygen levels in the fermentation medium are one of the factors responsible for the metabolic activity of the microorganism, according to Hongzhang et al. (2002). In addition, the authors state that high levels of air can reduce the humidity of the fermentation medium, impairing the production process. This was also verified in the present work, which reached reductions of moisture between 1.1 % (for the experiments without air supply and medium containing soybean oil) and 25.9 % (120 mLair.gmedium-1.h-1 in medium with soybean oil).
During fermentations, the experiments with120 mLair.gmedium-1.h-1 presented the fastest sporulation, possibly indicating higher cell growth. This fast sporulation, however, did not provide higher production of lipase. Thus, the enzyme production does not have a direct relation-ship with the microbial growth under the studied conditions. Elibol and Ozer (2000) studied the influence of aeration on the production of lipase by Rhizopus arrhizus and found that enzyme production presented an intrinsic relationship with the aeration rate and not with cell growth.
The highest lipolytic activities were found in 80 mLair.gmedium-1.h-1 for all nutritional conditions used in the experiments. Messias et al. (2009) also reported the influence of aeration on production of lipase by Botryosphaeria ribis EC-01. Another observation from those researchers is the influence of the source used for enzyme production. This same result was also reported by Kumar et al. (2011) in the production of lipase from Penicillium chrysogenum.
The best lipolytic activities were found in the experiments with soybean oil, thereby showing its synergistic effect on the production of lipase. Bresciani et al. (2014) and Colla et al. (2010) stated that the production of lipolytic enzymes by microorganisms can be induced by the type of oil present in the medium. According to Dominguez et al. (2003), lipase production can be influenced by the hydrocarbon type.
The best results were found in relatively short fermentation times, showing that there is no need for very long times for lipase production in this solid state fermentation.
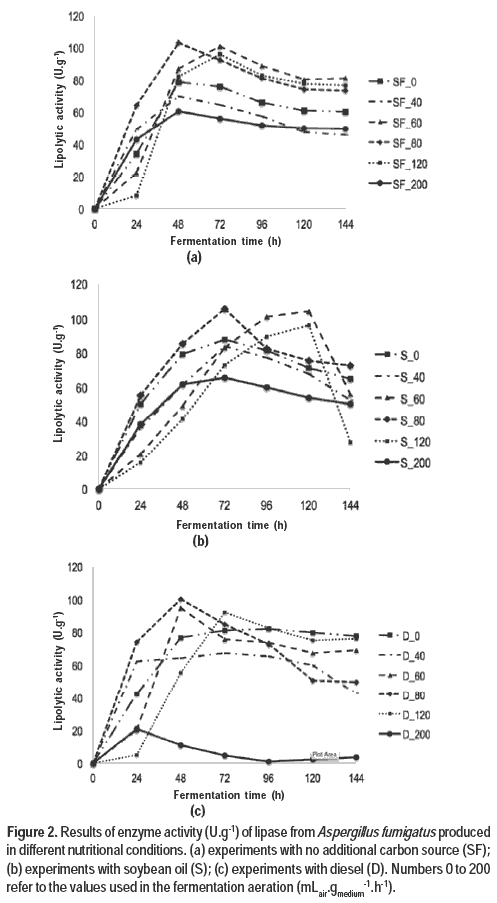
Figure 3 shows the cluster analysis for the different cultivation conditions evaluated.
It is possible to observe a differentiation in block of the results from the experimental conditions of cultivation. Given that cluster analysis is a process of partition of a heterogeneous population into several homogeneous subgroups, the elements are grouped according to their similarity. This classificatory analysis verified the existence of three groups of experiments with different similarities. The results did not show a direct relationship between the composition and aeration of the medium, but the use of 80 mLair.gmedium-1.h-1 allowed higher production of lipase, followed by the use of 60 and 120 mLair.gmedium-1.h-1 in cultures with no additional carbon source, 0, 40 and 60 mLair.gmedium-1.h-1 with soybean oil and 0 to 120 mLair.gmedium-1.h-1 using diesel as observed in Group 2. No significant differences (p <0.05) were found among the results of lipolytic activity from experiments in the same group, however, difference is observed among Groups 1, 2 and 3. This can be explained by the technique of block differentiation that was used, since it is known that the elements are grouped according to their similarity.
The measure of the difference was obtained from the values of the similarity matrix, showing that the observed differences among the three groups are a function of the influence of the aeration and exerts additional carbon source in the fermentation process.
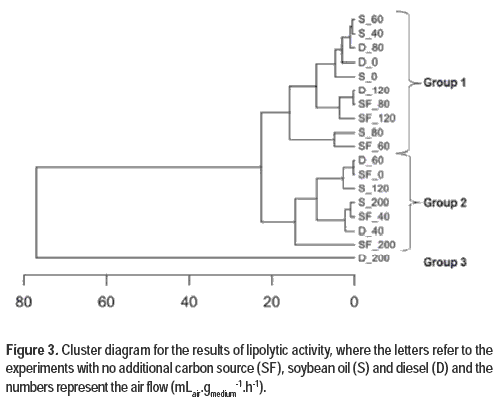
When evaluating the best results for the different aerations, it is observed that 80 mLair.gmedium-1.h-1 showed significantly higher lipolytic activity (p <0.05). The results can be observed in Figure 4, and the highest results were 103.13 U.g-1 using 80 mLair.gmedium-1.h-1 in 48 h of fermentation, in experiments without additional carbon source, and 105.76 U.g-1 with 80 mLair.gmedium-1.h-1 in 72 h, in the experiments with soybean oil, and 100.19 U.g-1 was found when using 80 mLair.gmedium-1.h-1 for 48 hours of fermentation in experiments with diesel.
Mohseni et al. (2012) evaluated agricultural residues for lipase production with Aspergillus niger, and found activities of up to 142 U.g-1. Shangguan et al. (2012), using the technique of gene expression of A. fumigatus, reached 54100 U.g-1 and Martins et al. (2008) found results ranging between 18.61 and 129.50 U.g-1 when evaluating co-production of lipase and biosurfactant production by A. fumigatus in rice hulls and in defatted rice bran.
The results of this study show how promising the utilization of agro-industrial residues as substrates in solid state fermentation can be, providing an alternative and value-addition to these residues from rice industry.
Conclusions
Among the aeration conditions used in this work, it is observed that the provision of 80 mLair.gmedium-1.h-1 can significantly increase the production of lipase by Aspergillus fumigatus. The best results were 103.13, 105.76 and 100.19 U.g-1 during 48, 72 and 48 h of fermentation, for the experiments with no additional carbon source plus soybean and diesel oils, respectively. The experiments showed no relationship between the results of lipolytic activity and aeration conditions, however, it was possible to verify the existence of three distinct groups regarding the similarity of the results. According to the results, this study suggests an economical alternative for the production of lipase solid state with Aspergillus fumigatus and residues from rice industry.
References
AOAC. 1995. Official Methods of Analysis of International. 16th ed. Arlington : AOAC.. 2v.
Bresciani FR, Santi L, Macedo AJ, Abraham WR, Vainstein MH, Beys-Da-Silva W. 2014. Production and activity of extracellular lipase from Luteibacter sp. Annals of Microbiology, 64(1): 251 – 258.
Burkert JFM, Maugeri F, Rodrigues MI. 2004. Optimization of extracellular lipase production by Geotrichum sp. using factorial design. Bioresource Technology, 91: 77 – 84.
Cammarota MC, Freire DMG. 2006. A review on hydrolytic enzymes in the treatment of wastewater with high oil grease content. Bioresource Technology, 97: 2195 – 2210.
Carvalho PO, Contesini FJ, Ikegaki M. 2006. Enzymatic resolution of (R,S)-ibuprofen and (R,S)-ketoprofen by microbial lipases from native and commercial sources. Brazilian Journal of Microbiology, 37(3): 329 – 337.
Carvalho PO, Contesini FJ, Bizaco R, Macedo GA. 2005. Kinetic Properties and Enantioselectivity of the lipases produced by four Aspergillus species. Food Biotechnology, 19(3): 183 – 192.
Castilho LR, Medronho RA, Alves TLM. 2000. Production and extraction of pectinases obtained by solid state fermentation of agroindustrial residues with Aspergillus niger. Bioresource Technology, 71: 45 – 50.
Castro HF, Anderson WA. 1995. Fine chemicals by biotransformation using lipases. Química Nova, 18: 544 – 554.
Ciafardini G, Zullo BA, Iride A. 2006. Lipase production by yeasts from extra virgin olive oil. Food Microbiology, 23: 60 – 67.
Colla LM, Rizzardi J, Pinto MH, Reinehr CO, Bertolin TE, Costa JAV. 2010. Simultaneous production of lipases and biosurfactants by submerged and solid-state bioprocesses. Bioresource Technology, 101: 8308 – 8314.
Diaz JCM, Rodríguez JA, Roussos S, Cordova J, Abousalham A, Carriere F, Baratti J. 2006. Lipase from the thermotolerant fungus Rhizopus homothallicus is more thermostable when produced using solid state fermentation than liquid fermentation procedures. Enzyme and Microbial Technology, 39: 1042 – 1050.
Domínguez A, Costas M, Longo MA, Sanromán A. 2003. A novel application of solid state culture: production of lipases by Yarrowia lipolytica. Biotechnology Letters, 25: 1225 – 1229.
Elibol M, Ozer D. 2000. Influence of oxygen transfer on lipase production by Rhizopus arrhizus. Process Biochemistry, 36: 325 – 329.
Hasan SDM, Costa JAV, Sanzo AVL. 1998. Heat transfer simulation of solid state fermentation in a packed-bed bioreactor. Biotechnology Techniques, 12(10): 787 – 791.
Hiol A, Jonzo MD, Rugani N, Druet D, Sarda L, Comeau LC. 2000. Purification and characterization of an extracellular lipase from a thermophilic Rhizopus oryzae strain isolated from palm fruit. Enzyme and Microbial Technology, 26: 421 – 430.
Hita E, Robles A, Camacho B, González PA, Esteban L, Jiménez MJ, Muñío MM, Molina E. 2009. Production of structured triacylglycerols by acidolysis catalyzed by lipases immobilized in a packed bed reactor. Biochemical Engineering Journal, 46: 257 – 264.
Hongzhang C, Fujian X, Zhonghou T, Zuohu L. 2002. A Novel Industrial-Level Reactor with Two Dynamic Changes of Air for Solid-State Fermentation. Journal of Bioscience and Bioengineering, 93(2): 211 – 214.
Kumar S, Katiyar N, Ingle P, Negi S. 2011. Use of evolutionary operation (EVOP) factorial design technique to develop a bioprocess using grease waste as a substrate for lipase production. Bioresource Technology, 102: 4909 – 4912.
Liu R, Jiang X, Mou H, Guan H, Hwang H, Li X. 2009. A novel low-temperature resistant alkaline lipase from a soda lake fungus strain Fusarium solani N4-2 for detergent formulation. Biochemical Engineering Journal, 46: 265 – 270.
Macêdo GA, Park YK, Pastore GM. 1997. Partial purification and characterization of an extracellular lipase from a newly isolated strain of Geotrichum sp. Journal of the Brazilian Society for Microbiology, 28: 90 – 95.
Martins VG, Kalil SJ, Costa JAV. 2008. Co-produção de lipase e biossurfactante em estado sólido para a utilização em biorremediação de óleos vegetais e hidrocarbonetos. Química Nova, 31(8): 1942 – 1947.
Messias JM, Costa BZ, Lima VMG, Dekker RF, Resende MI, Krieger N, Barbosa AM. 2009. Screening Botryosphaeria species for lipases: Production of lipase by Botryosphaeria ribis EC-01 grown on soybean oil and other carbon sources. Enzyme and Microbial Technology, 45: 426 – 431.
Mohseni S, Najafpour G, Vaseghi Z, Mahjoub S. 2012 Solid state fermentation of agricultural residues for lipase production in a tray-bioreactor. World Applied Sciences Journal, 16: 1034 – 1039.
Naqvi SHA, Dahot UD, Khan YK, Xu JH, Rafiq M. 2013. Usage of sugar cane bagasse as an energy source for the production of lipase by Aspergillus Fumigatus. Pakistan Journal of Botany, 45: 279 – 284.
Shangguan JJ, Fan LQ, Ju X, Zhu QQ, Wang FJ, Zhao J, Xu JH. 2012. Expression and Characterization of a Novel Enantioselective Lipase from Aspergillus fumigatus. Applied Biochemistry and Biotechnology, 168: 1820 – 1833.
Somashekar BR, Divakar S. 2007. Lipase catalyzed synthesis of l-alanyl esters of carbohydrates. Enzyme and Microbial Technology, 40(4): 299 – 309.
Wang PY, Chen YJ, Wu AC, Lin YS, Kao MF, Chen JR, Ciou JF, Tsai SW. 2009. (R,S)-Azolides as Novel Substrates for Lipase-Catalyzed Hydrolytic Resolution in Organic Solvents. Advanced Synthesis & Catalysis, 351(14-15): 2333 – 2341.
You Li C, Cheng CY, Chen TL. 2004. Fed – batch production of lipase by Acinetobacter radioresistens using Tween 80 as the carbon source. Biochemical Engineering Journal, 19: 25 - 31.












 Curriculum ScienTI
Curriculum ScienTI