Servicios Personalizados
Revista
Articulo
Links relacionados
Compartir
Agrociencia (Uruguay)
versión impresa ISSN 1510-0839versión On-line ISSN 2301-1548
Agrociencia Uruguay vol.19 no.1 Montevideo jun. 2015
Effect Of Soil Waterlogging Stress On The Physiological Performance Of Seeds And On The Productivity Of Rye Plants
Marques Gehling Vânia1, Pedó Tiago2, Garbin Martinazzo Emanuela3, Zanatta Aumonde Tiago4, Amaral Villela Francisco4
1 Federal University of Pelotas UFPel / FAEM. University Campus - PO Box 354 - CEP 96001-970, Pelotas – RS. E-mail: vaniagehling@hotmail.com
2 Federal University of Pelotas
3 Federal University of Rio Grande
4 Federal University of Pelotas
Recibido: 19/7/14 Aceptado: 31/1/15
Summary
The objectives of this study were to evaluate the effect of waterlogging on seed physiological performance and plant productivity in rye. Our study consisted of three treatments: 1) no waterlogging, 2) a single waterlogging period, and 3) double waterlogging period. Plant productivity was evaluated from the number of spikes per plant, the number of seeds per plant, the number of seeds per spike, and seed yield. Seeds were exposed to a germination test and seed physiological quality was evaluated from the seed germination rate, initial germination rate, germination speed index, electric conductivity, shoot length, primary root length, shoot dry matter and primary root dry matter, and weight per 1000 seeds. The seeds derived from plants not exposed to waterlogging showed a higher germination rate in a shorter period of time and higher germination speed index than those derived from plants that were exposed to waterlogging; however, the weight per 1000 seeds was lower and seedlings had longer shoots and primary roots. The plants that were not exposed to waterlogging had a higher number of spikes per plant, higher number of seeds per plant, higher number of seeds per spike, and better seed yield per plant. Therefore, it was concluded that waterlogging has a negative effect on seed physiological performance in rye and that plant productivity is reduced under long-term waterlogging.
Keywords: SECALE CEREALE L., ABIOTIC STRESS, SEED PHYSIOLOGICAL QUALITY, PLANT PRODUCTIVITY
Efecto estresante del anegamiento del suelo sobre el rendimiento fisiológico de semillas y sobre la productividad de plantas de centeno
Resumen
El objetivo de este trabajo fue evaluar el efecto del anegamiento del suelo sobre el comportamiento fisiológico de las semillas y la productividad de las plantas de centeno. Nuestro estudio consistió en tres tratamientos: 1) sin anegamiento; 2) un período de anegamiento; 3) dos períodos de anegamiento. La productividad de la planta se evaluó a partir del número de espigas por planta, el número de semillas por planta, el número de semillas por espiga y el rendimiento de las semillas. Las semillas fueron sometidas a una prueba de germinación para evaluar la calidad fisiológica de acuerdo a la tasa de germinación, tasa de germinación inicial, índice de velocidad de germinación, conductividad eléctrica, longitud del tallo, longitud de la raíz primaria, materia seca de la raíz y del tallo y peso de mil semillas. Las semillas de plantas no expuestas al anegamiento del suelo alcanzaron mayor porcentaje de germinación en menos tiempo y registraron mayores valores del índice de velocidad de germinación. Sin embargo, presentaron un menor peso de mil semillas, y las plántulas registraron mayor longitud del tallo y de la raíz primaria. Las plantas no expuestas al anegamiento tuvieron mayor número de espigas por planta, mayor número de semillas por planta, mayor número de semillas por espiga, y mejor rendimiento de semilla por planta. Por lo tanto, se concluye que el anegamiento del suelo en largos períodos afecta negativamente el rendimiento fisiológico de la semilla y la productividad de la planta de centeno.
Palabras clave: SECALE CEREALE L., ESTRÉS ABIÓTICO, CALIDAD FISIOLÓGICA DE LA SEMILLA, PRODUCTIVIDAD DE LA PLANTA
Introduction
Rye (Secale cereale L.) is an alternative winter crop in southern Brazil that differs from other winter cereals because of its acclimation capacity in unfavorable weather conditions and versatility; it is used as forage or cover crop (Fontaneli et al., 2009) and is suitable both for human consumption and animal feed. In Brazil, the rye acreage planted in the 2012/2013 growing season was more than 2000 ha, reach- ing a total production of 3,700 tons and an average yield of more than 1600 kg×ha-1 (Conab, 2013).
In Brazil, approximately 28×106 ha are flooded annually, and these soils are alluvial and hydromorphic (Magalhães et al., 2005). Rio Grande do Sul is an underutilized region of approximately 5.4×106 ha with lowland soils (Embrapa, 2005), where the primary crop is rice, and the land is also used for livestock production (Marchezan et al., 2002).
Waterlogging limits oxygen supply to the roots because it prevents or drastically reduces gas exchange between the radicular system and the pore spaces causing hypoxia or anoxia, respectively (Mattos et al., 2005; Zabalza et al., 2009). These two conditions cause energy metabolism dysfunction, decrease ATP synthesis, and reduce growth and productivity of cultivated plants (Horchani et al., 2008; Van Dongen et al., 2011). It is also possible that they lead to reduced physiological performance of seeds derived from plants exposed to waterlogging.
It is essential to study crop species with high-commercial value that have a similar cultivation system as irrigated rice mechanisms that help them tolerate waterlogging (Gazolla-Neto et al., 2012). Therefore, farming under waterlogging conditions is hard because of abiotic stress, which has a negative effect not only on plant productivity but also on the performance of the produced seeds.
In Brazil, the effect of waterlogging on plant growth and yield has been studied extensively in crops such as soybean and corn (Amarante et al., 2007; Coelho et al., 2013) but not in rye. The objective of this study was to evaluate the effect of waterlogging on the physiological performance of seed and productivity in rye.
Material and Methods
The effect of waterlogging on plant productivity was tested in a chapel greenhouse with a North-South orientation (31° 482 S 52° 242 W). The Pelotas Microregion has a humid and subtropical climate (Köppen climate classification, Cfa) with high-temperatures and well-distributed precipitation during the summer.
Seeds of ‘BRS Serrano’ rye cultivar were sown in 10-L black polyethylene containers filled with sieved soil that was collected from the A1 horizon of a solodic haplic eutrophic Planosol in the Pelotas Microregion (Streck et al., 2008). Fertilization and liming were performed seven and 60 days before sowing, respectively, following results of past soil analysis and recommendations of the Commission of Chemistry and Soil Fertility (Commissão de Química e Fertilidade do Solo, 2004).
Our study consisted of three treatments: 1) no waterlogging; soil moisture was maintained at field capacity; 2) a single waterlogging period, applied 45 days after seedling emergence (DAE) during the vegetative stage of tillers; 3) double waterlogging period, applied 45 DAE during the vegetative stage of tillers and 76 DAE during the booting stage of seeds.
Drainage holes were drilled into the bottom of the polyethylene containers to facilitate the flow of excess water and ensure maintaining soil moisture at field capacity. Field capacity, which was determined by the voltage table method (Embrapa, 1997), was used to define the level of moisture in the no-waterlogging treatment and establish the waterlogging treatments. In the latter, a 20-mm layer of water covered the soil surface for three days, which was achieved by placing a non-perforated black polyethylene container under the experimental container that also inhibited gas exchange and soil aeration. The non-perforated container was removed three days later allowing water drainage and consequently, moisture level to return to field capacity.
At the end of the cultivation cycle, plants were harvested and seeds were separated manually and taken to the Seed Analysis Laboratory, Phythotecnical Department, Agronomy College Eliseu Maciel, Federal University of Pelotas, where they were kiln-dried as described by Peske and Villela (2012). After drying, seeds were stored in a cold room as described by Baudet and Villela (2012). Analysis of productivity and physiological seed quality were performed following tests and evaluation methods as described below:
a) Productivity: data on the number of spikes per plant, the number of seeds per plant, and the number of seeds per spike were counted manually from four subsamples of four plants in each experimental unit and repeated six times. Seed yield per plant was obtained from the total weight of harvested seeds and values adjusted to a 13 % moisture basis (Piccinin et al., 2013).
b) Seed germination rate: seeds were staggered in two longitudinal parallel lines on the upper side of a roll made with Germitest® type paper and moistened with distilled water at a volume equal to 2.5 times its dry weight. Paper rolls with seeds were placed in the refrigerated incubator at 20 °C. Data on seed germination were collected seven days after sowing from four subsamples of 50 seeds in each experimental unit and repeated six times. Results were expressed as a percentage of the total number of seeds following the Rules of Seed Analysis (Brasil. Ministério da Agricultura, Pecuária e Abastecimento, 2009).
c) Initial germination rate: data on the number of normal seedlings were collected four days after sowing and results were expressed as a percentage of the total number of seeds following the Rules of Seed Analysis (Brasil. Ministério da Agricultura, Pecuária e Abastecimento, 2009).
d) Germination speed index (GSI): data on the number of germinated seeds (minimal radicle protrusion between 3 and 4 mm) were collected on a daily basis until the number of germinated seeds was stabilized as described by Nakagawa (1999).
e) Shoot and primary root length: seeds were staggered in two longitudinal parallel lines on the upper side of a roll made with Germitest® type paper and moistened with distilled water at a volume equal to 2.5 times its dry weight. Paper rolls with seeds were placed into the incubator at 20 °C. Data on shoot and primary root lengths were collected seven days after sowing from four subsamples of 20 seedlings in each experimental unit by using a millimeter ruler. The length of the shoot was determined as the distance between the insertion of the basal portion of the primary root and the apex of the shoot, while the length of the primary root length was determined as the distance between the apical and the basal part of the primary root. Results were expressed in mm×seedling-1.
f) Shoot and primary root dry matter: seedlings were dried in a forced air oven at 70 ± 2 °C, and water content was monitored gravimetrically. Data on shoot and primary root dry matter were collected from four subsamples of 20 seedlings in each experimental unit by using an analytical balance. Results were expressed as mg×seedling-1.
g) Weight per 1000 seeds: data were collected from four subsamples of 1000 seeds in each experimental unit and repeated six times by using an analytical balance. Results were expressed in grams following the Rules for Seed Analysis (Brasil. Ministério da Agricultura, Pecuária e Abastecimento, 2009).
h) Electrical conductivity: seeds of pre-measured weight were placed separately in polyethylene containers filled with 75 mL deionized water and kept at a temperature range between 20 and 25 °C. Electrical conductivity was measured under different soaking periods (3, 6, and 24 hours) by using the mass method as described by the International Seed Testing Association (Hampton and TeKrony, 1995). Data on electrical conductivity were collected from four subsamples of 50 seeds in each experimental unit by using a conductivity meter (Digimed Model DM-32). Results were expressed as µS×cm-1 ×g-1 of seeds.
The experimental design was completely randomized with six repetitions. Data were analyzed for normality and homoscedasticity. Analysis of variance was also performed in conjunction with Tukey’s test to identify the significant dif-ferences in means at a 95 % confidence level (Dias and Barros, 2009).
Results and Discussion
Germination rate, initial germination rate, and GSI of seeds derived from plants that were not exposed to waterlogging were higher than those derived from plants exposed to a single or double waterlogging period (Table 1).

A relatively higher germination rate usually corresponds to a high number of normal seedlings, whereas relatively higher initial germination rate and GSI is associated with the effective reorganization of the membrane systems and enhanced hydrolysis, translocation, and resource allocation to growth (Peske et al., 2012). Seeds with high vigor produce seedlings with better initial performance, which is associated with higher seed yield in the field (Peske et al., 2012).
Waterlogging has a negative effect on seed physiological performance due to the formation of a wet microclimate close to the soil surface (Vernetti, 2009). Ludwig (2010) reported the negative effect of waterlogging on different developmental stages of soybean plants and showed the reduced physiological performance of seed derived from plants exposed to waterlogging. Therefore, the plant’s ability to tolerate hypoxia is associated with its capacity for oxygen transport from shoots to roots (Bartlett and James, 1993).
The weight per 1000 seeds derived from plants that were exposed to double waterlogging was higher than those derived from plants that were not exposed to waterlogging or exposed to a single waterlogging (Table 1). Colmer and Voesenek (2009) reported that waterlogging causes an energy crisis for the plants due to stomatal closure, which is associated with the accumulation of sugars and starch in their seeds. Furthermore, waterlogging leads to the death of vegetative tissues, that may induce the formation of new tillers with high capacity for CO2 assimilation.
Waterlogging also reduces the rate of vegetative growth and the total number of flowers per plant (Runge and Odell, 1960). Thus, it should be noted that plants exposed to waterlogging may have a high rate of empty seeds because of the reduced flow. During plant development, preferential flow may occur, either during the post-fertilization phase or the filling of seeds, due to organs that are responsible for the constitution of a strong and definite flow (Pedó et al., 2013). Reduced flow may result in the formation of relatively denser and heavier seeds, because either the amount of drainage is low or the assimilation of nutrients is high.
Although the reduced weight per 1000 seeds is considered to be associated with low seed vigor, initial germination rate and GSI were higher in seeds that were derived from plants not exposed to waterlogging. Low vigor observed in seeds derived from plants exposed to waterlogging is probably due to the alteration of physiological, biochemical, or cytological attributes and not due to the lack of reserves in the seed. Therefore, seed deterioration may be better explained by the catalytic inefficiency of enzymes, the production of toxic compounds, and the inability of cell membranes to reorganize completely (Baudet and Villela 2012). It can also be associated with the reduction in starch, protein, carbohydrates, amino acids availability, and the negative effect of waterlogging on the catalytic efficiency of acid phosphatase (Santos et al., 1989).
The average values of shoot dry matter of produced seedlings derived from plants that were not exposed to waterlogging and from those exposed to double waterlogging were higher than those derived from plants exposed to a single waterlogging (Table 2). On the other hand, the average values of root dry matter did not differ significantly between the treatments (Table 2). These results show that the effect of waterlogging on assimilates partitioning between shoots and roots was quantitatively different.

The shoot and primary root of produced seedlings derived from plants that were not exposed to waterlogging were longer than those derived from plants that were exposed to waterlogging (Table 2). Seedling growth is associated with an increased physiological activity; therefore, seedlings with a well-developed radicular system may be more competitive during early development (Castro et al., 2008). Moreover, relatively higher shoot and root dry matter and length may be associated with greater capacity for resource mobilization and allocation to seedling growth, while increase in dry mat-ter and stagnation of weight with length reduction can be attributed to the fact that cell expansion is not followed by carbon accumulation (Burgos et al., 2004).
It is noteworthy that under short-term waterlogging or at the beginning of waterlogging, a metabolic acclimation occurs in plants, which involves the production of anaerobic stress proteins that help them to tolerate hypoxia (Irfan et al., 2010). However, long-term waterlogging leads to oxidative stress (Sairam et al., 2008), metabolic imbalance that has a negative effect on the development of plant structures and cellular mechanisms during the maturation process, and the physiological performance of seeds and seedlings.
The number of spikes was higher in plants that were not exposed to waterlogging than those exposed to a single waterlogging, which were also higher than those exposed to double waterlogging (Table 3). Similar results were observed for the number of seeds per plant and the number of seeds per spike; however, the latter did not differ significantly between the plants that were exposed to single and double waterlogging (Table 3). Seed yield per plant was higher in plants that were not exposed to waterlogging, and those exposed to a single waterlogging yielded better than those exposed to double waterlogging (Table 3).
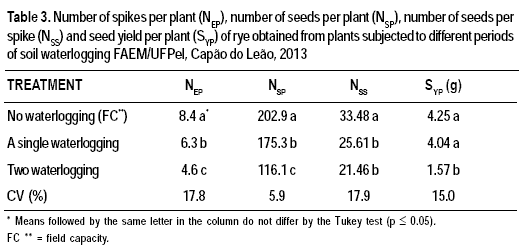
Waterlogging has a negative effect on growth, development, and plant production, due to the reduced speed of oxygen transport to the roots (Horchani et al., 2009), the closure of stomata, the decrease of photosynthetic activity (Colmer and Voesenek, 2009), the induction of ethylene biosynthesis, and the loosening of cell walls (Komatsu et al., 2011; Taiz and Zeiger, 2013).
Camargo et al. (2001) reported that waterlogging increases the production of organic acids, which in higher concentrations can inhibit plant growth, tillering, and nutrient absorption. Thomas and Costa (2010) reported that waterlogging during the vegetative stage has a negative effect on plant development and consequently on seed yield potential (Thomas and Costa, 2010), while Cho and Yamakawa (2006) reported that the duration of waterlogging is also negatively associated with plant productivity in soybean.
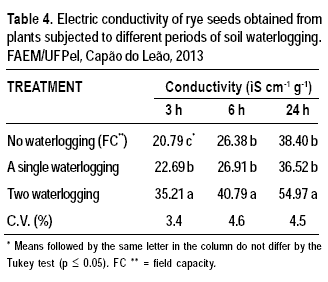
Electric conductivity of seeds derived from plants that were not exposed to waterlogging was lower than when derived from plants that were exposed to single or double waterlogging (Table 4). Increased electric conductivity, which is positively associated with waterlogging duration, may be explained by the inability of cell membranes to reorganize completely, and along with other physiological and biochemical factors may possibly lead to reduced physiological performance of seeds derived from plants exposed to waterlogging. Vieira et al. (2002) reported that low electrical conductivity is associated with low release of exudates and indicates a high physiological potential and increased ability of cell membranes to reorganize. Extravasation of resource is negatively associated with the translocation and allocation of CO2 and other compounds to seedling growth (Vanzolini and Nakagawa, 1999), which leads to reduced seedling performance.
Conclusion
Our results showed that waterlogging has a negative effect on seed physiological performance in rye and that plant productivity is reduced under long-term waterlogging. Nevertheless, reaction to waterlogging may be genotype-dependent; therefore, additional studies that will incorporate different rye genotypes are necessary to better understand the effect of waterlogging on seed physiological performance and plant productivity in rye. The effect of waterlogging stress in specific phenological periods in rye plants should also be studied in future research.
References
Amarante, L. do2007Amarante L, Colares DS, Oliveira ML, Zenzen IL, Badinelli PG, Bernardi E. 2007. Teores de clorofilas em soja associada simbioticamente com diferentes estirpes de Bradyrhizobium sob alagamento. Revista Brasileira de Biociências, 5: 906 – 908.
Bartlett RJ, James BR. 1993. Redox chemistry of soil. Advances in Agronomy, 50: 151 – 208.
Baudet LM, Villela FA. 2012. Armazenamento de sementes. En: Peske ST, Villela FA, Meneguello GE [Eds.]. Sementes : Fundamentos Científicos e Tecnológicos, 3ed. Pelotas : Editora rua Pelotas. pp. 481 – 527.
Brasil. Ministério da Agricultura, Pecuária e Abastecimento. 2009. Regras para análise de sementes. Brasília : MAPA. 395p.
Burgos NR, Talbert RE, Kim KS, Kuk YI. 2004. Growth inibition and root ultrastructure of cucumber seedlings exposed to allelochemicals from rye. Journal of Chemical Ecology, 30(3): 671 – 690.
Camargo FA, Zonta E, Santos G de A, Rossielo ROP. 2001. Aspectos fisiológicos e caracterização de toxidez a ácidos orgânicos voláteis em plantas. Ciência Rural, 31(3): 523 – 529.
Castro PRC, Kluge RA, Sestari I. 2008. Manual de fisiologia vegetal: fisiologia de cultivos. Viçosa : Editora Ceres. 864p.
Cho J, Yamakawa T. 2006. Effects on Growth and Seed Yield of Small Seed Soybean Cultivars of Flooding Conditions in Paddy Field. Journal of the Faculty of Agriculture, 51(2): 189 – 193.
Coelho CCR, Neves MG, Oliveira LM, Conceição AGC, Okumura RS, Oliveira Neto CF. 2013. Biometria em plantas de milho submetidas ao alagamento. Agroecossistemas, 5(1): 32 – 38.
Colmer TD, Voesenek LACJ. 2009. Flooding tolerance: suits of plant traits in variable environments. Functional Plant Biology, 36: 665 – 681.
Comissão de Química e Fertilidade do Solo. 2004. Manual de adubação e de calagem : para o Estado do Rio Grande do Sul e Santa Catarina. 10th ed. Porto Alegre : Sociedade Brasileira de Ciência do Solo. 400p.
Conab. 2013. Acompanhamento da Safra Brasileira : Grãos : Safra 2012/2013 : Décimo Levantamento : Julho/2013. Brasília : Conab [On line]. Cited January 10th 2014. Available from: http://www.conab.gov.br/OlalaCMS/uploads/arquivos/13_07_09_09_04_53_boletim_graos_junho__2013.pdf.
Dias LAS, Barros WS. 2009. Biometria Experimental. Viçosa : Suprema Gráfica e Editora. 408p.
Embrapa. 2005. Cultivo do Arroz Irrigado no Brasil [On line]. Sistemas de Produção, 3. Cited February 4th 2015. Available from: http://sistemasdeproducao.cnptia.embrapa.br/FontesHTML/Arroz/ArrozIrrigadoBrasil.
Embrapa. 1997. Manual de métodos de análise de solo. 2nd ed. Rio de Janeiro : CNPS. 212p.
Fontaneli RS, Fontaneli RS, Santos HP, Nascimento Junior A, Minella E, Caierão E. 2009. Rendimento e valor nutritivo de cereais de inverno de duplo propósito: forragem verde e silagem ou grãos. Revista Brasileira de Zootecnia, 38(11): 2116 – 2120.
Gazolla-Neto A, Aumonde TZ, Pedó T, Olsen D, Villela FA. 2012. Níveis de umidade do solo de várzea e seus efeitos sobre a emergência e crescimento inicial de plântulas de soja. Informativo Abrates, 22(2): 28 – 31.
Hampton JG, TeKrony DM. 1995. Handbook of vigour test methods. 3rd ed. Zürich : ISTA. 117p.
Horchani F, Khayati H, Raymond P, Brouquisse R, Aschi-Smiti S. 2009. Contrasted effects of prolonged root hypoxia on tomato root and fruit (Solanum lycopersicum) metabolism. Journal of Agronomy and Crop Science, 195(4): 313 – 318.
Horchani F, Gallusci P, Baldet P, Cabasson C, Maucourt M, Rolin D, Aschi-smiti S, Raymond P. 2008. Prolonged root hypoxia induces ammonium accumulation and decreases the nutritional quality of tomato fruits. Journal of Plant Physiology, 165(13): 1352 – 1359.
Irfan M, Hayat S, Hayat Q, Afroz S, Ahmad A. 2010. Physiological and biochemical changes in plants under waterlogging. Protoplasma, 241: 3 – 17.
Komatsu S, Thibaut D, Hiraga S, Kato M, Chiba M, Hashiguchi A, Tougou M, Shimamura S, Yasue H. 2011. Characterization of a novel flooding stress-responsive alcohol dehydrogenase expressed in soybean roots. Plant Molecular Biology, 77: 309 – 322.
Ludwig MP. 2010. Desempenho agronômico e qualidade de sementes de soja produzida em solo de várzea alagada [Doctoral Thesis]. Pelotas : Faculdade de Agronomia Eliseu Maciel, Universidade Federal de Pelotas. 115p.
Magalhães PC, Coelho CHM, Gama EEG, Borém A. 2005. Avaliação dos ciclos de seleção da variedade BRS 4154 – Saracura para tolerância ao encharcamento do solo. Sete Lagoas : Embrapa. 4p. (Circular Técnica ; 67).
Marchezan E, Vizzotto VR, Rocha MG, Moojen EL, Silva JHS. 2002. Produção animal em várzea sistematizada cultivada com forrageiras de estação fria submetidas a diferentes níveis de adubação. Ciência Rural, 32(2): 303 – 308.
Mattos JLS, Gomide JA, Huaman CAMY. 2005. Crescimento de Espécies do Gênero Brachiaria sob Alagamento em Casa de Vegetação. Revista Brasileira de Zootecnia, 34(3): 765 - 773.
Nakagawa J. 1999. Testes de vigor baseados no desempenho das plântulas. In: Krzyzanoswki FC, Vieira RD, França Neto JB. [Eds.]. Vigor de sementes: conceitos e testes. Londrina : ABRATES. pp. 1 – 24.
Pedó T, Aumonde TZ, Lopes NF, Villela FA, Mauch CR. 2013. Análise comparativa de crescimento entre genótipos de pimenta cultivados em casa de vegetação. Bioscience Journal, 29(1): 125 – 131.
Peske ST, Villela FA. 2012. Secagem de sementes. In: Peske, ST, Villela FA, Meneguello GE [Eds.]. Sementes : Fundamentos Científicos e Tecnológicos. 3rd. ed. Pelotas : Editora rua Pelotas. pp. 371 – 419.
Peske, ST, Villela FA, Meneguello GE. 2012. Sementes : Fundamentos Científicos e Tecnológicos. 3rd. ed. Pelotas : Editora rua Pelotas. 573p.
Piccinin GG, Braccini AL, Dan LGM, Bazo GL, Hossa KR, Ponce RM. 2013. Rendimento e desempenho agronômico da cultura do trigo em manejo com Azospirillum brasilense. Revista Agrarian, 6(22): 393 – 401.
Runge E, Odell RT. 1960. The relation between precipitation temperature and the yield of soybeans on the agronomy south farm. Agronomy Journal, 52(5): 245 – 247.
Sairam RK, Kumutha D, Ezhilmathi K, Deshmukh PS, Srivastava GC. 2008. Physiology and biochemistry of waterlogging tolerance in plants. Biologia Plantarum, 52: 401 – 412.
Santos DSB dos, Santos FBG dos, Gomes A da S, Pauletto EA, Schuch LA. 1989. Avaliação da qualidade fisiológica de sementes de soja em função de níveis de umidade no solo diferenciados. En: XVII Reunião Anual de Pesquisa de Soja da Região Sul; 24 – 27 julho; 1989; Porto Alegre, Rio Grande do Sul, Brasil. Porto Alegre : UFRGS pp. 227 – 227.
Streck EV, Kämpf N, Dalmolin RSD, Klamt E, Nascimento PC, Schneider P, Giasson E, Pinto LFS. 2008. Solos do Rio Grande do Sul. 2nd ed. Porto Alegre : EMATER/RS; UFRGS . 222p.
Taiz L, Zeiger E. 2013. Fisiologia vegetal. 5th ed. Porto Alegre : Artmed. 954p.
Thomas AL, Costa JA. 2010. Soja : manejo para alta produtividade de grãos. Porto Alegre : Evangraf. 243p.
Van Dongen JT, Gupta KJ, Ramírez-Aguilar SJ, Araújo WL, Nunes-Nesi A, Fernie AR. 2011 Regulation of respiration in plants : a role for alternative metabolic pathways. Journal of Plant Physiology, 168: 1434 – 1443.
Vanzolini S, Nakagawa J. 1999. Teste de condutividade elétrica em sementes de amendoim : efeitos de teor de água inicial e de período de embebição. Revista Brasileira de Sementes, 21(1): 46 – 52.
Vernetti Junior FJ. 2009. Soja : resultados de pesquisa na Embrapa Clima Temperado. Pelotas: Embrapa Clima Temperado. 78 p. (Embrapa Clima Temperado. Documentos ; 273).
Vieira RD, Penariol AL, Perecin D, Panobianco M. 2002. Condutividade elétrica e teor de água inicial de sementes de soja. Pesquisa agropecuária brasileira, 37(9): 1333 – 1338.
Zabalza A, Dongen JTV, Froehlich A, Oliver SN, Faix B, Gupta KJ, Schmälzlin E, Igal M, Orcaray L, Royuela M, Geigenberger P. 2009. Regulation of respiration and fermentation to control the plant internal oxygen concentration. Plant Physiology, 149: 1087 - 1098.














