Servicios Personalizados
Revista
Articulo
Links relacionados
Compartir
Agrociencia (Uruguay)
versión impresa ISSN 1510-0839versión On-line ISSN 2301-1548
Agrociencia Uruguay vol.13 no.1 Montevideo 2009
Comunicación Breve
Establishment of micropropagation and cell suspension culture conditions on Achyrocline flaccida (Weinm.) DC. (Asteraceae)
Establecimiento de suspensiones celulares de Achyrocline flaccida (Weinm.) DC. (Asteraceae) y condiciones para su micropropagación
Bonnecarrère, V.1; Berná, L.2; Castillo, A.1
1Instituto Nacional de Investigación Agropecuaria. Ruta 48 km 10, Rincón del Colorado, Canelones, Uruguay.
2 Laboratory of Animal Physiology and Evolution, Stazione Zoologica Anton Dohrn, Napoli, Italy.
Recibido: 17/3/09 Aceptado: 24/8/09
Summary
Achyrocline flaccida (Weinm.) DC. (Asteraceae) is a medicinal plant species, commonly known as yellow marcela. It is a rich source of flavonoids and other secondary metabolites with antioxidant properties. Their inflorescences are used as remedies in folk medicine for the treatment of a variety of human ailments. In fact, the permanence of this species is threatened by the increased interest of medicinal herb collectors. Thus, techniques which could provide vegetative propagated material for commercial use are necessary, and in vitro-propagation is a valuable method for producing large numbers of genetically uniform, pathogen-free plants in a short time. Moreover, the production of Achyrocline secondary metabolites is crucial for research and commercial large scale production since they are controllable systems and easy to scale up. Besides productive aims, cell culture suspensions are valuable tools to investigate metabolic pathways involved in secondary metabolites synthesis and to discover new bioactive molecules. The aims of this study were the optimization of a method to propagate in vitro plant of A. flaccida and the establishment of cell suspension cultures to determine the optimal culture conditions in order to improve cell growth as a first step toward secondary metabolites production. It was concluded that DKW without growth regulators is the optimal medium for micropropagation of this species. Friable callus formation was optimized in MS supplied with 0.5 mg L-1 2,4-D while cell suspensions were better obtained and maintained in DKW supplied with 1mg L-1 2,4-D.
Key words: growth regulators, in vitro culture, friable callus
Resumen
Achyrocline flaccida (Weinm.) DC. (Asteraceae), comúnmente conocida como marcela amarilla, es una especie de importancia medicinal debido a su alto contenido de flavonoides y otros metabolitos secundarios con propiedades antioxidantes. Sus inflorescencias son usadas para el tratamiento de un gran número de enfermedades, lo que ha conducido a considerar esta especie en riesgo de extinción debido al gran interés de los colectores de hierbas medicinales. Por esta razón, es muy importante el establecimiento de técnicas de propagación vegetativa con fines comerciales, que permitan la producción de un gran número de plantas, libre de patógenos, y en un corto plazo. Además, la producción directa de metabolitos secundarios de Achyrocline es crucial para la investigación y la producción con fines comerciales ya que son sistemas controlados donde es posible aumentar fácilmente la escala de producción. Además de ser utilizados con fines comerciales, los cultivos de células presentan grandes ventajas como sistema para el estudio de vías metabólicas y el descubrimiento de nuevas biomoléculas. Los objetivos de este trabajo fueron la optimización de métodos de micropropagación in vitro de A. flaccida y el establecimiento de suspensiones celulares para determinar las condiciones óptimas de crecimiento celular como primer paso hacia la producción de metabolitos secundarios. Se determinó que DKW sin reguladores del crecimiento es el medio adecuado para la micropropagación de esta especie. La obtención de callos friables se optimizó en medio MS suplementado con 0.5 mg L-1 de 2,4-D mientras que las suspensiones celulares se obtuvieron y mantuvieron en medio DKW suplementado con 1 mg L-1 de 2,4-D.
Palabras clave: reguladores del crecimiento, cultivo in vitro, callo friable
Introduction
Achyrocline flaccida (Weinm.) DC. (Asteraceae) is a medicinal plant species, native of Brazil, Paraguay, Argentina and Uruguay (Bandoni, 2000). It is commonly known as yellow marcela and their inflorescences are used as remedies in folk medicine for the treatment of a variety of human ailments. They are used as medicinal tea and are widely known for their choleretic, antispasmodic and digestive actions (Toursarkissian, 1980; Kadarian et al., 2002). Other medical properties have been related to Achyrocline species, such as: antioxidant, anti-microbial, antiherpetic, anti-HIV, hepatoprotective, antihyperglycemic, immunostimulant, insecticidal and molluscicidal (Gutkind et al., 1981; Wagner et al., 1985; Garcia et al., 1990; Puhlmann et al., 1992; Desmarchelier et al., 1998; Garcia et al., 1999; Hnatyszyn et al., 1999; Carney et al., 2002; Gugliucci and Menini, 2002; Ruffa et al., 2002).
Phytochemical analyses of Achyrocline spp. demonstrated that they are a rich source of secondary compounds, including terpenoids, phenylpropanoids and flavonoids (Ferraro et al., 1981; Mesquita et al., 1986; Broussalis et al., 1989). Initially, chemistry studies identified many poliphenols such as: galangin, galangin-3-mehtyl ether, quercetin, quercetin-3-methyl ether, caffeic acid, two esters of calleryanin (3,4-dihydroxybenzyl alcohol 4-glucoside) and protocatechuic acid (Ferraro et al., 1981 and Broussalis et al., 1989). Mesquita et al. (1986) isolated two new flavonoids: 5-hydroxy-3,6,7-trimethoxyflavone (alustin) and 5,7,8-trimethoxyflavone which are considered very rare flavonoids. A. flaccida is the only Achyrocline species with epoxybutoxy derivatives, which are difficult to find in other plant species (Broussalis et al., 1989). Recently, Retta et al. (2008) studied quantitative and qualitative composition of essential oil in A. flaccida and demonstrated that it is a rich source of a-pinene and b-caryophyllene.
Considering analytical and biological studies, A. flaccida has demonstrated to be a valuable source of pharmaceutical and cosmetically interesting molecules. For these reasons this species is threatened by the increased interest of medicinal herb collectors. Therefore, techniques which could provide vegetative propagated material for commercial use and for possible reintroduction are very important. In vitro-propagation is consideres a valuable method for producing large numbers of genetically uniform, pathogen-free plants in a short time. Moreover, the production of A. flaccida secondary metabolites in a high quantity and quality manner, is an important challenge in order to cover market demands. Cell suspension culture is a suitable alternative since they are controllable systems and feasible to scale up (Roberts and Shuler, 1997). The development of plant cell culture systems for the production of high value chemicals was driven by the possibility to manipulate the culture conditions to lead to high flux into secondary metabolic pathways, and then to be able to grow such cultures in large scale bioreactors (Sudha and Ravishankar, 2002). Important metabolites, such as paclitaxel (Taxol) are being successfully produced in cell suspension cultures using elicitors to increase product yield (Zhong, 2002). Besides productive aims, cell culture suspensions are helpful tools to investigate metabolic pathways involved in secondary metabolite synthesis and to discover new bioactive molecules.
The aim of this study was the optimization of a method to propagate in vitro plant cell growth as a first step toward secondary metabolite production.
Materials and methods
Plant material and surface sterilization
Seeds were collected from a population of A. flaccida situated at the nursery of medicinal and aromatic plants of the National Agricultural Research Institute (INIA) in Las Brujas, Canelones, Uruguay. They were surface sterilized by immersions on 20 % (v/v) hypochlorite solution for 15 min under continuous agitation. They were immediately rinsed three times with sterile distilled water and germinated on MS (Murashige and Skoog) medium (Murashige and Skoog, 1962).
In vitro culture and micropropagation
Cultures were initiated in 25 x 150 mm test tubes. Afterwards, they were transferred to 300 ml glass jars for shoot proliferation. Inorganic and organic media constituents, growth regulators, and their interactions were investigated.
Plants were micropropagated by cutting internodal segments which were located in new media. Three salt media were tested: MS, Driver and Kuniyuki Walnut (DKW) (Driver and Kuniyuki, 1984) and Woody Plant Medium (WPM) (Lloyd and McCown, 1981). The effect of cytokinin 6-benzylaminopurine (BAP) was evaluated by adding 0.3 mg.L-1 to each medium. All the media contained 30 g L-1 of sucrose and 8 g L-1 of agar for solidification. The pH was adjusted to 5.8 before autoclaving (1.1 kg cm-2 at 121º C for 20 min). Plants were grown at 22 ± 2º C with 16-hour photoperiod under 3 nmol m-2 s-1 photosynthetic photon flux. Growth was assayed after 4 weeks considering the fresh weight (FW), morphology and presence of plant hyperhydricity. FW data were analyzed with ANOVA.
Determination and optimization of callus induction medium
Calli were established by culturing segments leaves excised from aseptic seedlings. Leaves of five weeks old plants were cut into 0.5 cm2 segments and placed upside down in Petri- dishes containing MS solidified with 0.8 % agar (Agar Chile) and supplemented with casein (250 mg L-1), 2,4-dichlorophenoxyacetic acid (2,4-D) and kinetin (KIN). Three 2,4-D concentrations (0.5 mg L-1; 1.0 mg L-1; 1.5 mg L-1), two KIN concentrations (0.5 mg L-1; 1.0 mg L-1) and all possible combinations were tested. Each treatment contained 4 replications with 10 explants per replication. Cultures were maintained at 22 ± 2º C with 16-hour photoperiod under 3 nmol m-2 s-1 photosynthetic photon flux. After 4 weeks, callus number (percentage), friability and FW was determined. FW data were analysed with ANOVA.
Generation of cell suspensions and establishment of optimal cell growth conditions
Friable callus (1 g wet weight) was transferred to 50 ml of MS and DKW liquid medium in 300 ml Erlenmeyer flask closed with aluminium foil. The media was amended either with 1 mg L-1 2,4-D or 0.3 mg L-1 BAP in order to analyze different growth regulators. They were incubated under 16-hour photoperiod at 22 ± 2º C on an orbital shaker model SLR-25T (Ikeda Scientific Co. Ltd) at 120 rpm. Cell growth was determined by measuring cell suspension FW and dry weight (DW) every 3 days over 37 days. Three mL of culture were taken from each flask and filtered through a filter paper (Whatman ® No. 1) under vacuum suction. The DW was calculated after drying the samples at 65º C for 48 h. The values obtained are the mean of the three replicates.
Growth rates (µ) were calculated according to the equation for exponential growth cultures:
µ = ln (W1/W2)/t2-t1 (Singer et al., 1985).
Results and discussion
Plant in vitro culture and micropropagation
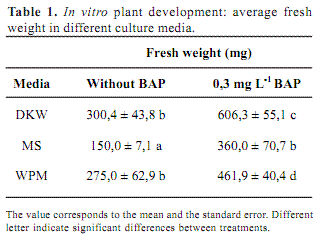
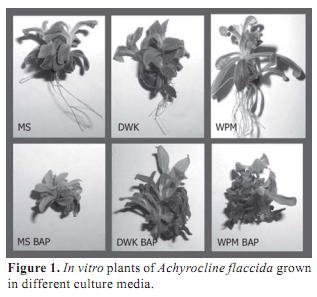
Considering A. flaccida seeds small size, the very thin tegument thickness and the hardness of the sterilization method it was expected very low germination rate. Nevertheless, 100 % of seed germination was obtained 10 days after disinfection and all plants were able to grow on different culture media. In order to asses the best growth medium, three parameters were considered: fresh weight, morphology and hyperhydricity. In all cases, plant fresh weight was higher in media supplied with BAP respect to the same medium without the regulator (Table 1). However, BAP induced shorter internodes, forming very compact structures and all plant showed high degree of hyperhydricity (Figure 1). Hyperhydricity, also known as vitrification, is a morphological, anatomical and physiological malformation that makes the plant tissue water-swollen (Paques and Boxus, 1987). The phenomenon has been correlated to water availability, microelements and/or hormonal imbalance in the tissue culture medium (Kataeva et al., 1991).
Media salt composition also affected plant development. The best plant performance was obtained in DKW compared to WPM and MS. Considering FW, DKW and WPM did not showed any differences but plants grown in DKW presented better structure. DKW and WPM contain lower concentration of nitrogen, either nitrate or ammonium, and potassium in comparison to MS. These concentrations resemble natural conditions where Marcela normally grows. Possibly high nitrogen or potassium concentrations inhibit plant development. In addition, the content of other nutrient such as calcium, and specially micronutrient as chloride and iodide, are considerable different among these media. Iodide and chloride are only present in MS and they may be the responsible of plant growth inhibition. In conclusion, the best salt medium for A. flaccida micropropagation is DKW without any plant regulator.
Establishment of cell suspension cultures
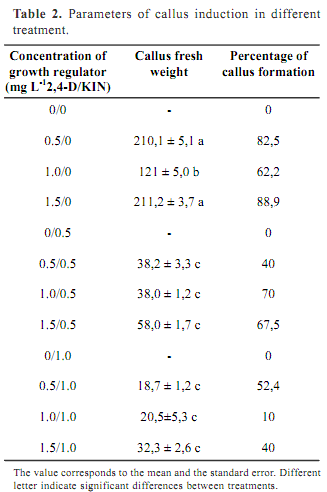
Despite of the results obtained with entire plants, it was not possible to get friable callus on DKW media. In fact, these results confirm that organogenesis and callus formation optimal conditions differ, since both physiological processes are completely different. The only media where friable callus were formed was MS. Consequently it was selected for callus formation. After 4 weeks callus were induced in all media containing 2,4-D and KIN. However, there were significant differences among treatment. Table 2 shows the percentages of callus formation and the average of callus FW per treatment. No friable callus was formed from explants cultured on MS without hormones and in all medium supplied with only KIN. On the contrary, all media containing 2,4-D, either 0,5 mg L-1, 1,0 mg L-1, or 1,5 mg L-1, induced friable callus formation. The best results were obtained in medium supplied only with 2,4-D regardless the concentration. The growth regulator 2,4-D has been cited as the best regulator to induce callus formation in Rubus idaeus (Borejsza-Wysoki and Hrazdin, 1994) and Orthosiphon stamineus (Lee and Chang, 2004).
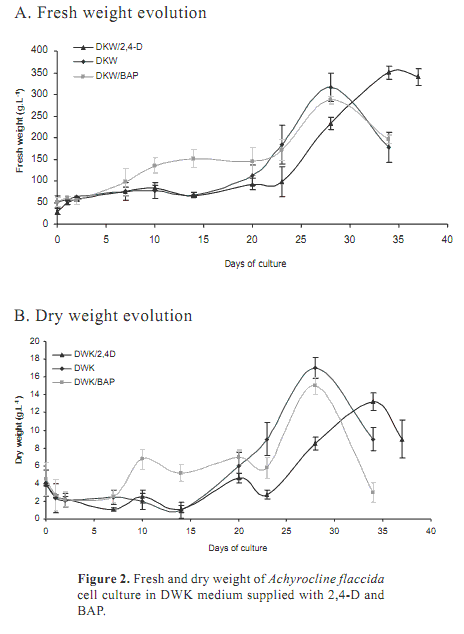
Friable callus were dispersed on liquid media in order to get cell suspensions. Normally, cell suspensions are established in the same media were callus are formed (Lee and Chang, 2004). However, in MS media, all suspension differentiates to roots (data not shown). Considering theses results, DKW was selected as medium for the establishment of cell culture. In this medium no tissue differentiation was registered and it was possible to establish continuous suspensions over one year.
Auxins, particularly 2,4-D, are commonly used for the establishment of cell suspension cultures (Szabados et al., 1991). The action of BAP cytokinin was also evaluated. Figure 2 shows the evolution of fresh and dry weight registered over 37 days. All media showed a very large latent phase, approximately 20 days. There were differences during the exponential phase. Media without regulators or supplied with BAP had greater growth rates (m) compared to DKW supplied with 2,4-D (mDKW=1.19, mDKW/BAP=1.02 and mDKW/2,4-D 0.99). However, in DKW supplied with 2,4-D the exponential phase was longer (10 days) and the FW at the end of the period was higher. This increase in FW is not correlated to DW, where suspensions containing 2,4-D showed the lowest values. It is known that 2,4-D induce the formation of soft callus by increasing the size of vacuoles (Borejsza-Wysoki and Hrazdin, 1994). The same phenomenon could happen in this suspension culture and it has to be considered for the production of metabolites which are secreted to the vacuole.
In summary, cell suspension cultures of A. flaccida could be established from friable callus produced by culturing leaf explants on MS medium supplied with 0.5 mg L-1 2,4-D. The optimal medium for cell suspension is DKW amended with 1 mg L-1 2,4-D.
This study is the preliminary work for upcoming experiments focused on secondary metabolite production by A. flaccida cell suspension cultures. To accomplish this aim it is necessary to evaluate different cell sources, as stems, roots and petiole. The type of explants has been cited as influencing callus formation (Lee and Chang, 2004), cell suspension and metabolite profile. Therefore optimal growth conditions and metabolite production have to be determined for all these explants, in order to compare their metabolic profile.
Acknowledgments
We thank the staff of the in vitro culture laboratory of the Biotechnology Unit and the nursery of medicinal and aromatic plants of the National Institute of Agriculture Research (INIA) in Las Brujas, Canelones, Uruguay.
References
Bandoni, L. 2000. Los Recursos Vegetales Aromáticos en Latinoamérica. Ed. Universidad Nacional de La Plata. La Plata, Argentina.
Borejsza-Eysocki, W. and Hrazdin, G. 1994. Establishment of callus and cell suspension cultures of raspberry (Rubus idaues cv. Royalty). Plant Cell, Tissue and Organ Culture. 37: 213-216.
Broussalis, A. M.; Ferraro, G. E.; Gurni, A. A. and Coussio, J. D. 1989. Aspectos fitoquímicos de especies Argentinas del género Achyrocline. Acta Farmaceutica Bonaerense, 8: 11-16.
Carney, J.; Krenisky, J.; Williamson, R. and Luo, J. 2002. Achyrofuran, a new antihyperglycemic dibenzofuran from the South American medicinal plant Achyrocline satureioides. Journal of Natural Products, 65: 203-205.
Desmarchelier, C.; Coussio J. and Ciccia G. 1998. Antioxidant and free radical scavenging effects in extracts of the medicinal herb Achyrocline satureioides (Lam.) D.C. (marcela). Braz. J. Med. Biol. Res., 31: 163-170.
Driver, D. and Kuniyuki, D. 1984. In vitro propagation of Paradox walnut rootstock. HortScience, 19: 507-509.
Ferraro, G.; Norbedo, C. and Coussio, J. 1981. Polyphenols from Achyrocline satureioides. Phytochemistry, 8: 2053-2054.
Garcia, G. H.; Campos, R. and De Torres, R. A. 1990. Antiherpetic activity of some Argentine medicinal plants. Fitoterapia, 6: 542-546.
Garcia, G.; Cavallaro, L.; Broussalis, A.; Ferraro, G.; Martino, V. and Campos, R. 1999. Biological and chemical characterization of the fraction with antiherpetic activity from Achyrocline flaccida. Planta Med., 65: 343-346.
Gugliucci A. and Menini T. 2002. Three different pathways for human LDL oxidation are inhibited in vitro by water extracts of the medicinal herb Achyrocline satureioides. Life Science, 71: 693-705.
Gutkind, G. O.; Martino, V. and Graña, N. 1981. Screening of South American plants for biological activities. 1. Antibacterial and antifungal activity. Fitoterapia, 52: 213-218.
Hnatyszyn, O.; Broussalis, A.; Herrera, G.; Muschietti, L.; Coussio, J.; Martino, V.; Ferraro, G.; Font, M.; Monge, A.; Martinez-Irujo, J. J.; Sanroman, M.; Cuevas, M. T.; Santiago, E. and Lasarte, J. J. 1999. Argentine plant extracts active against polymerase and ribonuclease H activities of HIV-1 reverse transcriptase. Phytotherapy Research, 13: 206-209.
Kadarian, C.; Broussalis, A. M.; Miño, J.; Lopez, P.; Gorzalczany, S.; Ferraro, G. and Acevedo, C. 2002. Hepatoprotective activity of Achyrocline satureioides (LAM) D.C. Pharmacol. Res., 45: 57-61.
Kataeva, N. V.; Alexanandrova, I. G.; Butenko, R. G. and Dragavtceva, E. V. 1991. Effect of applied and internal hormones on vitrification and apical necrosis of different plants cultured in vitro. Plant Cell, Tissue and Organ Culture, 27: 149-154.
Lee, W. L. and Chang, L.K. 2004. Establishment of Orthosiphon stamineus cell suspension culture for cell growth. Plant Cell, Tissue and Organ Culture, 78: 101-106.
Lloyd, G. and MC Cown, B. 1981. Commercially feasible micropropagation of mountain laurel, Kalmia latifolia, by use of shoot-tip culture. Comb. Proc. Intl. Plant Prop. Soc., 30: 421-427.
Mesquita, A.; Correa, D.; De Paula, A.; Guedes, M. and Gottlieb, O. 1986. Flavonoids from four Compositae species. Phytochemistry, 5: 1255-1256.
Murashige, T. and Skoog, F. 1962. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant, 15: 473-497.
Paques, M. and Boxus, P. 1987. Vitrification: review of literature. Acta Hort., 66: 212:155.
Puhlmann, J.; Knaus, U.; Tubaro, L.; Schaefer, W. and Wagner, H. 1992. Immunologically active metallic ion-containing polysaccharides of Achyrocline satureioides. Phytochemistry, 31: 2617-2621.
Retta, D.; Gattuso, M.; Gattuso, S.; Di Leo Lira, P.; van Baren, C.; Ferraro, G. and Bandoni, A. 2008. Essential oil composition of Achyrocline flaccida (Weinm.) DC. (Asteraceae) from different locations of Argentina. Bioch. System. and Ecol., 36: 877-881.
Roberts, S. C. and Shuler, M. L. 1997. Large-scale plant cell culture. Current Opinion in Biotechnology, 8:154–159.
Ruffa, M. J.; Ferraro, G.; Wagner, M. L.; Calcagno, M. L.; Campos, R. H. and Cavallaro, L. 2002. Cytotoxic effect of Argentine medicinal plant extratcs on human hepatocellular carcinoma cell line. J. Ethnopharmacology, 79: 335-339.
Singer, S. R. 1985. Analyzing growth in cell cultures. I. Calculating growth rates. Can. J. Bot., 60: 233-237.
Sudha G. and Ravishankar, G. A. 2002. Involvement and interaction of various signaling compounds on the plantmetabolic events during defense response, resistance to stress factors, formation of secondary metabolites and their molecular aspects. Plant Cell, Tissue and Organ Culture, 71: 181-212.
Szabados, L.; Mroginski, L. A. y Roca, W. M. 1991. Suspensiones Celulares: Descripción, Manipulación y Aplicaciones. En Cultivos de Tejidos en la Agricultura. Fundamentos y Aplicaciones. Publicación CIAT, 151: 173-210.
Toursarkissian, M. 1980. Plantas medicinales de la Argentina. Ed. Hemisferio Sur, Buenos Aires, Argentina.
Wagner, H.; Proksch, A.; Riess-Maurer, I.; Vollmar, A.; Odenthal, S.; Stup, H.; Jurcic, K.; Le Turdu, M. and Heur, Y. H. 1985. Immunostimulant action of polysaccharides (heteroglycans) from higher plants. Arzneimittelforschung, 7: 1069-1105.
Zhong, J. J. 2002. Plant cell culture for production of paclitaxel and other taxanes. Journal of BioScience and Bioengineering, 94: 591-599.