Servicios Personalizados
Revista
Articulo
Links relacionados
Compartir
Odontoestomatología
versión impresa ISSN 0797-0374versión On-line ISSN 1688-9339
Odontoestomatología vol.25 no.42 Montevideo 2023 Epub 01-Dic-2023
https://doi.org/10.22592/ode2023n42e330
Actualizaciones
Histological - Molecular Characteristics of Dental Cement. The non-fibrillar matrix and its role in the origin, maintenance and tissue regeneration
1 Especialista en Periodoncia. Facultad de Odontología, Universidad de la República.
2 Cátedra de Histología. Facultad de Odontología, Universidad de la República.
Cementum is a connective tissue with the particularity that its organic matrix ismineralized. Within its components it is possible to identify the cementoblasts, the cementocytes and in certain conditions the cementoclasts. In the organic matrix there are two components, one fibrillar, represented mainly by collagen type I, and another non-fibrillar, which includes diverse molecules capable of producing a varied number of functions. Being a mineralized connective, the hydroxyapatite articulates with the organic matrix to produce this mineralization.
The objective of this work is to update the histological-molecular characteristics of cement, and especially the non-fibrillar extracellular matrix components, and how these contribute to maintenance and tissue regeneration.
Keywords: Cementum/ non-fibrillary matrix/ Regeneration
El cemento es un tejido conjuntivo con la particularidad de que su matriz orgánica esta mineralizada. Dentro de sus componentes es posible identificar a las células donde podemos reconocer a los cementoblastos, los cementocitos y en ciertas condiciones los cementoclastos. En la matriz orgánica hay dos componentes, uno fibrilar representado principalmente por el colágeno tipo I, y otro no fibrilar el cual incluye moléculas diversas capaces de producir una variada cantidad de funciones. Al ser un conectivo mineralizado, la hidroxiapatita articula con la matriz orgánica para producir esta mineralización.
El objetivo del presente trabajo es realizar una actualización de las características histológico - moleculares del cemento, y en especial de los componentes de matriz extracelular de tipo no fibrilar, y como estas aportan al mantenimiento y regeneración tisular.
Palabras clave: Cemento Dental / Matriz no fibrilar / Regeneración
O cemento é um tecido conjuntivo com a particularidade de sua matriz orgânica ser mineralizada. Dentro dos seus componentes encontramos as células onde podemos identificar os cementblastos, os cementócitos e em certas condições os cementclastos. Na matriz orgânica encontramos dois componentes, um fibrilar, representado principalmente pelo colágeno tipo I, e outro não fibrilar, que inclui diversas moléculas capazes de produzir um variado número de funções. Por ser um conjuntivo mineralizado, encontramos a hidroxiapatita que se articula com a matriz orgânica para produzir esta mineralização.
O objetivo deste trabalho é atualizar as características histológico-moleculares do cimento, e principalmente dos componentes da matriz extracelular não fibrilar, e como estes contribuem para o manutenção e regeneração tecidual.
Palavras-chave: Cemento/ Matriz nao fibrilar/ Regeneracao
Introduction
Cementum is a connective tissue located in the tooth root, characterized by its organic matrix's ability to mineralize. As a connective tissue, it consists of three basic components: cells, a fibrillar matrix, and non-fibrillar matrix components, with the unique feature that the organic matrix is mineralized and could be considered a fourth structural component (1. Among the cells, cementoblasts are specialized in the secretion and synthesis of all matrix components; they are topographically located on the tissue surface, considered by some authors as cells of the periodontal ligament (PL), a tissue with which they are intimately related. Morphologically, they present a globular to cuboidal aspect, with a central single nucleus and organoids in variable quantity according to the degree of functionality; they are more abundant in cells with greater metabolic activity (1. Cementoblasts can become embedded in the cement matrix and immersed in cavities called cementoplasts, becoming cementocytes, altering their morphology to flattened cells, and intracellularly transforming into cells with very few organoids2.
As mentioned, when considering the extracellular matrix, there are two well-defined phases: the fibrillar phase, mainly represented by type I collagen molecules, and the non-fibrillar phase, generally defined by different substances such as proteins associated with mineralization processes, growth factors, among others. They could be grouped according to their functions, nature, or participation in different tissue processes 2.
This work aims to provide an update on the histological-molecular characteristics of cementum, particularly the non-fibrillar extracellular matrix components, and their contributions to tissue maintenance and regeneration.
Methodology
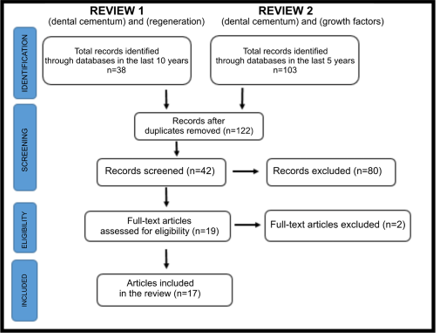
A literature search was conducted using the PubMed MEDLINE, Google Scholar, and LILACS databases, incorporating articles from the last five years involving human subjects. The initial search employed the descriptors (dental cementum) and (regeneration), and in a subsequent search, (dental cementum) and (growth factors) were utilized. The search was executed in August and September 2022, resulting in 122 articles in the first phase. After a comprehensive review, 42 articles were screened, from which 19 were assessed for eligibility, and 17 were included. The inclusion criteria were based on the relevance of the articles concerning their connection to the outlined objectives. The drafting of the final paper required incorporating three references related to disciplinary texts essential for describing specific generic aspects of the topic. (Fig. 1)
Description
Dental cementum is a connective tissue with a mineralized organic matrix. Four structural elements can be identified: cells, two phases of the organic matrix (fibrillar and non-fibrillar), and the inorganic phase represented by hydroxyapatite and other inorganic components.
The non-fibrillar organic matrix comprises 5 groups of substances that have the particularity of participating in various physiological processes of the tissue 2. The molecules are as follows:
1. Bone Morphogenetic Proteins (BMP)
BMPs belong to the superfamily of transforming growth factors B (TGF-B). They are divided into at least 4 subgroups according to the functions performed by each one of them 2, as follows:
BMP 2/BMP 4;
BMP 5 (also known as osteogenic protein 1), 6, 7, 8a, and 8b;
BMP 9 and 10;
BMP 12, 13, and 14 (also known as cartilage-derived BMP 1).
They act through transmembrane serine and threonine protein kinase receptors. Several BMPs, such as BMPs 2, 4, and 7, promote the differentiation of preosteoblasts and cementoblast precursor cells. Additionally, in some experimental models and certain clinical situations, they have induced periodontal regeneration 3.
A study by Hakki et al. 4 demonstrated the effects of BMP7 on cementoblasts, proving its regulation of gene expression associated with mineralized tissue by stimulating cementoblast-mediated biomineralization in vitro 5. There is evidence that it is capable of promoting cementogenesis when in contact with the extracellular matrix of dentin, inducing the differentiation of cementoblasts and matrix mineralization 6. BMP2 inhibits fibroblast differentiation and mineralization; therefore, it cannot promote cementum and periodontal ligament regeneration 6. Likewise, Ripamonti U. 7 emphasized the importance of BMP7, together with the BMP2 molecule, in participating in the cementogenesis process at the root level.
Yong et al. 8 observed how molecules such as ciliary neurotrophic factor (CNTF) can be evidenced. This molecule, through the inhibition of BMPs, particularly BMP 7, can inhibit biological processes such as cementogenesis and trigger autophagy. This generates inputs that could lead to the facilitation of inflammatory root resorption during orthodontic tooth movement. Moon et al. 9 have suggested that Hedgehog (Hh) signaling is involved in cementoblast differentiation, as well as in the regulation of BMP7, presenting itself as a possible therapeutic target for periodontal treatment and regeneration.
2. Epithelial factors
The signaling molecules involved in the morphogenesis of the crown and root are the same among the epithelial factors. According to Nanci 3, these include the following:
While EMPs haven't been consistently identified throughout the root, the possibility that they may influence the differentiation of odontoblasts and cementoblasts in the early stages of root formation is not ruled out 3.
Amelogenin molecules, derived from enamel matrix, are clinically used to stimulate repair and regeneration. They are expressed in Hertwig’s Epithelial Root Sheath cells, odontoblasts, cementoblasts, and periodontal ligament cells. These proteins also have biological effects in cells of ectomesenchymal origin, such as those of the periodontal ligament and gingival fibroblasts 9. During cementoblast differentiation, they intervene in the adult periodontium to induce undifferentiated ectomesenchymal cells to the cementoblast phenotype (2. Amelogenin expression has also been found in hematopoietic stem cells, macrophages, megakaryocytes, rat brains, and myoepithelial cells. Amelogenin has a regulatory role in the recruitment and differentiation of monocytic bone marrow cells to become cells that resorb bone tissue matrix and cementum, such as osteoclasts and cementoclasts, respectively (2.
A mouse study conducted by Huang et al. (10 in 2009 had already provided insights into the action of Hertwig's epithelial root sheath (HERS) on cementum. HERS cells synthesize Cement Adhesion Proteins (CAP) and Cement Adhesion Protein 1 (CEMP 1), cells capable of participating in cementum production, with increased alkaline phosphatase (ALP) activity, favoring the formation of acellular or cell-free cementum 10.
HERS cells and epithelial rests of Malassez constitute a unique population in the periodontal ligament, playing a crucial and essential role in cementum repair. They have the potential to differentiate into cementoblasts through mesenchymal-epithelial transformation. A 2011 in vitro study by Nam et al. 11 demonstrated that HERS/ epithelial rests of Malassez contain primitive stem cells expressing epithelial stem cell markers. Studies suggest that epithelial rests of Malassez are epithelial stem cells capable of differentiating into epithelial or ectomesenchymal cells, thereby playing a significant role in periodontal repair and regeneration (12. By inducing the expression of CAP and CEMP1, Enamel Matrix Derivatives are believed to promote the differentiation of dental follicle or tooth sac cells into a cementoblast phenotype rather than an osteoblast phenotype. According to Kémoun et al. in 2007 13, dental follicle or tooth sac cells show positivity for CAP and CEMP1 when stimulated with EMD or BMP 2/7. CAP binds to fibronectin, but its binding to HA is much stronger. This protein selectively binds to periodontal ligament cells and is compatible with the binding of periodontal ligament cells to the root surface.
CAP's higher expression during the early stages of cementogenesis is related to its function of promoting cell proliferation. In contrast, CEMP1 assumes a more crucial role in regulating the mineralization process at this early stage of cementogenesis 12. CEMP1 facilitates the adherence of periodontal ligament cells and the proliferation and differentiation of cementoblasts rather than osteoblasts. In addition to promoting mineralized tissue formation, there are carbohydrates that bind to CEMP1. Glycosylation could impact the protein's function during mineralization since its anionic surface can bind Ca2+ ions, thereby regulating the growth of HA crystals. CEMP1 appears to be a phosphorylated protein, evidenced by cross-reactivity with antibodies against phospho-serine and phospho-threonine. Phosphate favors Ca2+ binding to the protein, and other mineralization-associated proteins, such as Sialoprotein and Osteopontin, are highly phosphorylated 9. It promotes the nucleation of Octacalcium Phosphate crystals 14. Octacalcium Phosphate intervenes in a transitory phase during crystal growth. In the case of small crystals, Octacalcium Phosphate transforms into HA through hydrolysis and is detectable only in large crystals. Both cementum proteins are crucial for their potential involvement in the histo-differentiation of periodontal stem cells, inducing differentiation and regulating the biological mineralization process associated with cementum formation. The synergistic collaboration between enamel-associated and cementum proteins in these processes is noteworthy. CEMP1 exhibits an affinity for hydroxyapatite (HA), influencing crystal morphology. It induces the formation of polymorphous crystals, making it necessary for their synthesis in a needle-shaped form and plays a significant role in biomineralization 9. Additionally, research demonstrates that a peptide derived from the N-terminus of CEMP1 promotes the differentiation of periodontal ligament cells toward a phenotype similar to mineralization. This bioactive peptide exhibits osteoinductive and osteogenic properties 12.
3. Matrix proteins for cell adhesion: Bone Sialoprotein and Osteopontin.
Two highly significant molecules in this category are Bone Sialoproteins (BSP) and Osteopontin (OPN). These multifunctional molecules play a crucial role in mineralization processes, particularly in the formation of cement during the development and repair of periodontal tissues 15. They are phosphoproteins that fill spaces generated during collagen assembly, and enable the widespread deposition of minerals throughout the collagen meshwork. Consequently, they regulate the growth and nucleation of hydroxyapatite (HA) crystals 15. These proteins contain Arginine, Glycine, and Aspartic Acid sequences, mediating cell adhesion to the developing root. The balance between the activities of these molecules may maintain the non-mineralized periodontal ligament between the cementum and alveolar bone.
In the periodontium, OPN is expressed by cells in close proximity to acellular or cell-free enclosed cementum and by cementocytes. BSP expression influences cementum formation and periodontal attachment by promoting mineralization in the root, enabling the anchoring of periodontal ligament fibers. During root formation, BSP is located in cells that cover the cementum surface, modulating the process of cementogenesis and intervening in the chemoattraction, adhesion, and differentiation of cementoblasts. Both OPN and BSP are believed to play a crucial role in differentiating progenitor cells into cementoblasts 16. Liu et al. demonstrated that cementoblast differentiation and cementum mineralization are facilitated through Wnt/β-catenin signaling, offering a potential strategy in periodontal regenerative therapy 17. Li et al. 18 showed how stress-induced expression significantly augmented OPN and other molecules in the periodontium, indirectly regulating the formation of cementoblasts.
4. Gla Proteins
Gla proteins are enriched with y-carboxyglutamic acid, an amino acid that binds to calcium. OCN (Bone Gla Protein) (bone gamma-carboxyglutamic acid protein) serves as a maturation marker for osteoblasts and cementoblasts, regulating the extent of mineralization2. This osteoblast-derived hormone also plays a role in regulating insulin secretion and energy expenditure. MGP (Matrix Gla protein), or matrix γ-carboxyglutamic acid protein, has been observed in periodontal tissues. Acting as a mineralization inhibitor, it could preserve the width of the periodontal ligament and prevent hypercalcification of the cementum surface. It is secreted by cementum-forming cells and incorporates into the mineralization front. Both proteins serve as negative regulators of mineralization, but to varying degrees, as OCN also inhibits the conversion of brushite to hydroxyapatite. In 2018, Yang et al.19 presented evidence that YAP1 can enhance mineralization by exerting its effects on cementoblasts.
5. Alkaline Phosphatase (ALP)
Alkaline Phosphatase (ALP) is a glycoprotein enzyme that hydrolyzes phosphate groups at alkaline pH and inhibits Pyrophosphatase, ATPase, and Protein phosphatase activity at neutral pH2. It exhibits high expression in periodontal ligament cells, playing a crucial role in phosphate metabolism and the formation of cementum in its various forms. One of its primary functions is the hydrolysis of inorganic pyrophosphate, an inhibitor of hydroxyapatite formation. Cementoblasts are sensitive to the extracellular matrix's inorganic pyrophosphate/inorganic phosphate levels. Alterations in ALP levels significantly impact osteoblast function and matrix mineralization, thus its pivotal role in bone tissue and cement mineralization is deduced20. A proposed strategy for achieving more predictable cementum regeneration involves reducing inorganic pyrophosphate by modulating the inorganic pyrophosphate/inorganic phosphate ratio in the periodontium, thereby increasing cementum neoformation.
Alkaline phosphatase, along with metalloproteinases, proteoglycans, and various growth factors (IGF, TGF-B, and platelet growth factor), are molecules within periodontal tissues that regulate diverse activities, including those of cementoblasts, both concerning their differentiation and activity. At the dentin-cement junction, a substantial presence of proteoglycans is evident, suggesting their potential involvement in the initial mineralization and adhesion of fibers in collaboration with other non-collagenous matrix proteins, such as BSP and OPN 3,9.
In vitro studies have demonstrated that ligament cells exhibiting ALP positivity also manifest higher expression levels of mineralization-related genes (BSP and OCN) than phosphatase-negative ligament cells. All these ALP properties may enable the development of therapies based on Cement Protein 1 for periodontal regeneration (3.
Growth factors represent a large family of polypeptide proteins that bind to cell receptors and guide cell behavior, such as cell attachment, cell survival, proliferation, chemotaxis, and differentiation. In this way, they achieve the growth of specific tissues. They are expressed in the tissue during its physiological remodeling or after trauma. Their production is regulated by gene expression and stem cell differentiation 5,6.
This table (table 1) summarizes some of the non-fibrillar matrix molecules that participate in various functions related to Cementogenesis:
| Molecule | Author(s) | Function |
|---|---|---|
| BMP2 | Ripamonti et al.7 | Cementum cells differentiation. |
| BMP7 | Yong et al. 4 | Stimulates biomineralization. |
| EMP | Nanci 3 | Cementoblasts differentiation. |
| Amelogenin | Cementoblasts and other connective tissue cells differentiation. | |
| CAP | Huang et al. 10 | Promotes proliferation. |
| CEMP1 | Kemoun et al. 13 Huang et al. 10 | Promotes mineralization. Cementoblasts differentiation. |
| BSP | Fu 15 | Promotes mineralization. |
| OPN | Li 16 | Promotes mineralization. |
| GLA Proteins | Yang 19 | Inhibit mineralization. |
| ALP | Sacramento 20 | Promotes mineralization. |
Discussion
There is diverse evidence regarding the functions performed by cementum extracellular matrix components, particularly the array of elements comprising the non-fibrillar portion of the matrix.
When considering the tissue's physiology, it becomes evident that BMP2 plays a role in cell differentiation, as indicated by Ripamonti et al. (7, EMP as by Nanci (3, and CEMP1 according to Huang et al. 10. Other studies related to participatory roles in proliferative activities, as reported by Huang et al. 10), further provide evidence of CAP's involvement. Several molecules contribute to mineralization, such as CEMP1 according to Huang et al. (10, BSP according to Fu 15, OPN according to Li 16, ALP according to Sacramento 20, and BMP7 according to Yong et al. 4. We have also found evidence of molecules that inhibit mineralization as expressed by Yang 19, namely, GLA proteins.
Additionally, proteins of the BMP family can regulate various cement-related aspects. Hakki 4) reports that BMP 7 controls the expression of several genes. Further studies offer more specificity regarding these functions; Carmagnola (6) and Smith 5 discuss the promotion of mineralization. Yong 8) adds to this evidence, suggesting that when other molecules, like CNTF, are present, they can inhibit the action of BMP. This family includes BMP2, linked to the differentiation of cementoblasts according to Ripamonti et al. (7.
Amelogenins, proteins produced by epithelial cells of diverse epithelia with odontogenic function, present significant evidence in the non-fibrillar matrix. Arzate 9) proposes their potential action on undifferentiated ectomesenchymal cells, and Garant (2 suggests their role in the formation of cementoblasts and other connective cells, such as hematopoietic stem cells, macrophages, megakaryocytes, rat brains, and myoepithelial cells. Additionally, they regulate the recruitment and differentiation of monocytic bone marrow cells, leading to the formation of cells like osteoclasts and cementoclasts, involved in resorbing bone tissue matrix and cementum, respectively (2.
Hertwig's epithelial root sheath (HERS), an epithelial structure active in root formation during odontogenesis, can synthesize CAP and CEMP1, inducing periodontal cells to produce biomineralization, as stated by Huang 10 and Arzate 9. CEMP1, as noted by Montoya, exhibits diverse activities, controlling mineralization, or having a bioactive role with osteogenic or osteoprogenitor properties.
One particular element that authors such as Nam et al. 11 showed was that HERS/ epithelial rests of Malassez contain primitive stem cells expressing epithelial stem cell markers. Montoya et al. (12) corroborated this finding, showing that epithelial rests of Malassez consist of epithelial stem cells with the ability to differentiate into epithelial or ectomesenchymal cells and have an important role in periodontal repair/regeneration.
Existing evidence affirms the participation of BSP and OPN in mineralization, as expressed by Fu (15. However, Li 16 suggests that BSP might also contribute to the differentiation of cementoblasts, highlighting the existence of molecules with polymodal actions within this tissue structure.
When examining mineralization, we also find Gla proteins, as indicated by Garant (2, Runx2, as mentioned by Rodriguez et al. (20, and OSX, as reported by Choi et al. However, factors that repress this function emerge, as demonstrated by Fu (15, who highlights the repression that REV-ERBs impose on OSX. This repression prevents the progression of mineralization processes, underscoring the necessity for a highly selective and intricate control mechanism in this process.
Alkaline phosphatase (ALP) is highly expressed in periodontal ligament cells, where it plays a role in phosphate metabolism and, consequently, in mineralization. As described by Sacramento et al. 20, it regulates the function of various cells, including osteoblasts and cementoblasts. These studies on these molecules could modulate cement neoformation, being involved in mineralization alongside BSP and OPN, as they state.
Alkaline phosphatase, alongside metalloproteinases, proteoglycans, and various growth factors (IGF, TGF-B, and platelet growth factor), are molecules within periodontal tissues that regulate various activities, including cementoblast differentiation and activity. In the dentin-cement junction, a large amount of proteoglycans is observed, and it is considered that they may be involved in the initial mineralization and adhesion of the fibers, along with other non-collagenous proteins of the matrix, such as BSP and OPN, as proposed by Nanci 3) and Arzate 9.
When considering tissue regeneration and the non-fibrillar matrix components of cement, we can evidence that, as Montoya 12 states, CEMP1 can play a role in the regulation of cement matrix mineralization, or EMP in the production of cement matrix as well as in the activation of resorptive processes of cement, as suggested by Garant (2) and Nanci 3. In experimental studies, Nanci 3) has shown that BMP2 is also important in the induction of regeneration.
In addition to the molecules of the non-fibrillar matrix, tissue regeneration can be favored by HERS cells, which could participate in particular phenomena such as the so-called mesenchymal epithelial transformation, as described by Nam 11 and Montoya 12. This is an element to be studied in greater depth and given careful consideration because of the therapeutic possibilities that could arise from them.
Conclusions
The significance of the non-fibrillar components of the cement matrix, whether in its origin (cementogenesis) or in tissue maintenance during various physiological scenarios arising from pathological or therapeutic phenomena, can be clearly demonstrated.
In addition to contributing conceptually, this work provides valuable insights for decision-making in dental clinics. These decisions are rooted in evidence and certainties, enabling the guidance of clinical practices based on a thorough understanding of the biological context.
Our findings confirm that tissue regeneration and cementum maintenance rely on both the composition of the extracellular matrix and the activities of Hertwig's epithelial root sheath cells and epithelial rests of Malassez; these are believed to have the potential to transform into mesenchymal cells, which, in turn, differentiate into cementoblasts to fulfill their specific functions.
REFERENCES
1. Gomez de Ferraris ME, Campos Muñoz A. Periodonto de inserción: cemento, ligamento periodontal y hueso alveolar. En: Histología, Embriología e Ingeniería Tisular Bucodental. Ciudad de México. Panamericana. 4 ed., 2019. p267- 299. [ Links ]
2. Garant P. Root Formation and Cementogenesis. Oral Cells and Tissues. 1era ed. Canada: Quintessence, 2003. p179-194. [ Links ]
3. Nancy A. Periodontium. Ten Cate's Oral Histology. 9 ed. Montreal. Elsevier, 2017. p193-217. [ Links ]
4. Hakki SS, Foster BL, Nagatomo KJ, Bozkurt SB, Hakki EE, Somerman MJ, Nohutcu RM. Bone morphogenetic protein-7 enhances cementoblast function in vitro. J Periodontol 2010: 81: 1663-1674. [ Links ]
5. Smith PC, Martínez C, Cáceres M, Martínez J. Research on growth factors in periodontology. Periodontol 2000. 2015; 67: 234-250 [ Links ]
6. Carmagnola D, Pellegrini G, Dellavia C, Rimondini L, Varoni E. Tissue engineering in periodontology: Biological mediators for periodontal regeneration. The International Journal of Artificial Organs.2019; 1-17 [ Links ]
7. Ripamonti U, Developmental pathways of periodontal tissue regeneration: Developmental diversities of tooth morphogenesis do also map capacity of periodontal tissue regeneration? J Periodontal Res ; 54(1): 10-26, 2019 Feb [ Links ]
8. Yong, J Gröger, S; Von Bremen, J; Ruf, S. Ciliary Neurotrophic Factor (CNTF) Inhibits In Vitro Cementoblast Mineralization and Induces Autophagy, in Part by STAT3/ERK Commitment. Int J Mol Sci ; 23(16)2022 Aug 18 [ Links ]
9. Arzate H., Zeichner-Davis M., Mercado-Celis G. Cementum proteins: role in cementogénesis, biomineralization, periodontium formation and regeneration. Periodontol. 2000. 2015; 65: 211-233. [ Links ]
10. Huang X, Bringas P Jr, Slavkin HC, Chai Y. Fate of HERS during tooth root development, Dev Biol. 2009 Oct 1;334(1):22-30. doi: 10.1016/j.ydbio.2009.06.034. Epub 2009 Jul 1. [ Links ]
11. Nam H, Kim J, Park J, Park JC, Kim JW, Seo BM, Lee JC, Lee G. Expression profile of the stem cell markers in human Hertwig's epithelial root sheath/Epithelial rests of Malassez cells. Mol Cells. 2011 Apr;31(4):355-60. doi: 10.1007/s10059-011-0045-3. Epub 2011 Feb 22. [ Links ]
12. Montoya G, Correa R, Arenas J, Hoz L, Romo E, Arroyo R, Zeichner- Davis M, Arzate H. Cementum protein 1-derived peptide (CEMP 1-p1) modulates hydroxyapatite crystal formation in vitro.J Pep Sci. 2019; 1-11. [ Links ]
13. Kémoun P, Laurencin-Dalicieux S, Rue J, Vaysse F, Roméas A, Arzate H, Conte-Auriol F, Farges JC, Salles. Localization of STRO-1, BMP-2/-3/-7, BMP receptors and phosphorylated Smad-1 during the formation of mouse periodontium. Tissue Cell. 2007 Aug;39(4):257-66. doi: 10.1016/j.tice.2007.06.001. Epub 2007 Jul 26. [ Links ]
14. Nuñez J, Vignoletti F, Caffesse R, Sanz M. Cellular therapy in periodontal regeneration. Periodontology 2000. 2019; 79 (1): 107-116 [ Links ]
15. Fu, L; Wang, M; Zhu, G; Zhao, Z; Sun, H; Cao, Z; Xia, H REV-ERBs negatively regulate mineralization of the cementoblasts. Biochem Biophys Res Commun ; 587: 9-15, 2022 01 08. [ Links ]
16. Li, Shengnan; Li, Fan; Zou, Shujuan; Zhang, Li; Bai, Yuxing. PTH1R signalling regulates the mechanotransduction process of cementoblasts under cyclic tensile stress. Eur J Orthod ; 40(5): 537-543, 2018 09 28. [ Links ]
17. Liu, S; Zhou, Y; Chen, Y; Liu, Y; Peng, S; Cao, Z; Xia, H. Bmal1 promotes cementoblast differentiation and cementum mineralization via Wnt/ß-catenin signaling. Acta Histochem ; 124(3): 151868, 2022 Apr. [ Links ]
18. Li, S; Li, F; Zou, S; Zhang, L; Bai, Y. PTH1R signalling regulates the mechanotransduction process of cementoblasts under cyclic tensile stress. Eur J Orthod ; 40(5): 537-543, 2018 09 28. [ Links ]
19. Yang, B; Sun, H; Song, F; Wu, Yu; Wang, J. Yes-associated protein 1 promotes the differentiation and mineralization of cementoblast. J Cell Physiol ; 233(3): 2213-2224, 2018 Mar. [ Links ]
20. Sacramento, CM; Assis, RI ; Saito, MT; Coletta, RD; Da Rocha Dourado, M; Sallum, EA; Nociti, FH; Viana Casarin, RC; Andia, DC; Silvério, KG. BMP-2 and asporin expression regulate 5-aza-dC-mediated osteoblast/cementoblast differentiation of periodontal dental ligament mesenchymal progenitor cells. Differentiation ; 124: 17-27, 2022. [ Links ]
Conflict of Interest Statement: The authors have no conflicts of interest in the publication of this article
Authorship and Contribution Statement: 1. Conception and design of the study 2. Data Acquisition 3. Data analysis 4. Results Discussion 5. Manuscript drafting 6. Approval of the final version of the manuscript. VT has contributed in 1, 2, 3, 4, 5, 6. AC has contributed in 5, 6. CD has contributed in 5, 6. GT has contributed in 1, 2, 3, 4, 5, 6.
Received: September 27, 2023; Accepted: November 12, 2023










 texto en
texto en