Servicios Personalizados
Revista
Articulo
Links relacionados
Compartir
Odontoestomatología
versión impresa ISSN 0797-0374versión On-line ISSN 1688-9339
Odontoestomatología vol.25 no.42 Montevideo 2023 Epub 01-Dic-2023
https://doi.org/10.22592/ode2023n42e329
Actualizaciones
Association between periapical lesions and diabetes mellitus
1 Departamento de Odontología preventiva y restauradora. Facultad de Odontología, Universidad de la República. migarchitorena@gmail.com
2 Departamento de Odontología preventiva y restauradora. Facultad de Odontología, Universidad de la República. ernesto.andrade1977@gmail.com
3 Departamento de Odontología preventiva y restauradora. Facultad de Odontología, Universidad de la República. adrifejodi@gmail.com
Periapical lesions are inflammatory processes that generate the resorption of mineralized tissues. In diabetic patients this process may be affected.
This work aims to identify the association between diabetes mellitus and periapical lesions and to know if diabetic patients have a higher prevalence and severity.
A comprehensive review of the available literature, of a narrative type, was carried out. The PubMed (Medline) and SciELO databases and the Timbó and Google Scholar resources were consulted. The exclusion criteria were: works prior to the year 2010 and case reports. Articles prior to the exclusion date were included, as they were considered relevant to the work.
Despite the fact that the scientific evidence is still insufficient and the design of the studies should be improved, an association between periapical lesions and diabetes mellitus has been demonstrated. This implies that diabetic patients could have a higher prevalence and severity of lesions.
Keywords: Apical periodontitis; periapical lesions; diabetes mellitus
Las lesiones periapicales son procesos inflamatorios que generan la reabsorción de los tejidos mineralizados. En pacientes diabéticos este proceso puede verse afectado.
Este trabajo tiene como objetivo identificar la asociación entre la diabetes mellitus y las lesiones periapicales y conocer si los pacientes diabéticos presentan mayor prevalencia y severidad.
Se realizó una revisión amplia de la literatura disponible, de tipo narrativa. Se consultaron las bases de datos PubMed (Medline) y SciELO y los recursos Timbó y Google Scholar. Los criterios de exclusión fueron: trabajos anteriores al año 2010 y reportes de caso Se incluyeron artículos anteriores a la fecha de exclusión por considerarse relevantes para el trabajo.
A pesar que la evidencia científica continúa siendo insuficiente y el diseño de los estudios debe mejorarse, se demuestra asociación entre lesiones periapicales y diabetes mellitus. Esto implica que los pacientes diabéticos podrían presentar mayor prevalencia y severidad de lesiones.
Palabras clave: Periodontitis apical; lesiones periapicales; diabetes mellitus
As lesões periapicais são processos inflamatórios que geram a reabsorção de tecidos mineralizados. Em pacientes diabéticos este processo pode ser afetado.
Este trabalho tem como objetivo identificar a associação entre diabetes mellitus e lesões periapicais e saber se os pacientes diabéticos apresentam maior prevalência e gravidade.
Foi realizada uma revisão abrangente da literatura disponível, do tipo narrativa. Foram consultadas as bases de dados PubMed (Medline) e SciELO e os recursos Timbó e Google Acadêmico. Os critérios de exclusão foram: trabalhos anteriores ao ano de 2010 e relatos de casos.Os artigos anteriores à data de exclusão foram incluídos por serem considerados pertinentes ao trabalho.
Apesar de as evidências científicas ainda serem insuficientes e o delineamento dos estudos precisar ser aprimorado, foi demonstrada uma associação entre lesões periapicais e diabetes mellitus. Isso implica que os pacientes diabéticos podem ter maior prevalência e gravidade das lesões.
Palavras chave: Periodontite apical; lesões periapicais; diabetes melito
Introduction
In periapical lesions (PL), endodontic treatment aims to create conditions conducive to tissue neoformation. Regeneration involves a process of tissue renewal with cells that share similar characteristics to those that were lost (1).
Apical regeneration is thus a process involving bone remodeling mechanisms (resorption/formation of bone tissue), which involve the participation of osteoclasts, osteoblasts, and osteocytes. It is noteworthy that the metabolism of these cells is affected by both local conditions and systemic factors (1).
Diabetes mellitus (DM) is a highly prevalent pathology in our country. The second national survey conducted by the Ministry of Public Health in 2013 on risk factors for noncommunicable diseases (2) revealed that the prevalence of diabetes is approximately 7.6% for the population aged 25 to 64 years. Moreover, prevalence increases with age. Notably, for the age range between 55 and 64 years, the estimate is 16.8%, with no significant differences between men and women.
In a cross-sectional descriptive study carried out at the Registration Service of the Faculty of Dentistry (UdelaR) between August 2015 and May 2016, a prevalence of 15.6% of diabetic patients was detected. This figure rises to 30.3% when considering patients over 65 years.(3)
The integrity of the immune system, tissue remodeling, and regulation in osteoclastic differentiation and activity could be altered in diabetic patients (4), determining susceptibility to PL.
In 2014, the European Society of Endodontics (ESE) organized a symposium titled “Relationship between apical periodontitis and general health.” The conclusions established a low level of evidence on this topic (5).
From the 1990s, and more prominently since 2000, "Periodontal Medicine" has evolved as a field focused on the relationship between periodontal disease (PD) and systemic diseases. Several epidemiological studies have established an association between PD and DM (6,7). Since PD and chronic apical inflammatory processes share several characteristics (both are oral polymicrobial infections with a microbiota predominantly composed of gram-negative anaerobic bacteria that trigger inflammatory processes), it could be assumed that PL could also be related to systemic diseases (8,9).
Similarly, the concept of "Endodontic Medicine" emerged(8). This term refers to the bidirectional relationship between endodontic infections and systemic diseases and is supported by evidence showing that systemic diseases influence the pathogenesis of endodontic infection, just as the latter can cause general health alterations(10).
Objectives:
This review work aims to analyze the association between diabetes mellitus and periapical lesions and to establish whether diabetic patients have a higher prevalence and severity of PL compared to patients without DM.
Method:
A comprehensive review of available narrative literature was conducted to identify papers addressing the relationship between DM as a systemic disease associated with PL, regarding prevalence and severity. PubMed (Medline) and SciELO databases, along with Timbo and Google Scholar resources, were consulted.
The search strategy was:
"periapical periodontitis"(MeSH Terms) OR "periapical"(All Fields) AND "periodontitis"(All Fields) OR "periapical periodontitis"(All Fields) OR "apical"(All Fields) AND "periodontitis"(All Fields) OR "apical periodontitis"(All Field) AND "diabetes mellitus"(MeSH Terms) OR "diabetes"(All Fields) AND "mellitus" (All Fields) OR "diabetes mellitus"(All Fields)
Exclusion criteria were: papers before 2010 and case reports.
37 articles were retrieved, including 25 primary documents (original articles) and 12 secondary (systematic reviews, meta-analyses, umbrella reviews).
The search was complemented by reading and tracking bibliography referenced in these articles, gathering a total of 46 documents published from 1986 to 2020. Articles prior to the exclusion date were included because they were considered relevant to the work.
Description
PL are inflammatory processes around the apex of the tooth root, primarily resulting from a microbial infection within the pulp. This inflammation leads to the resorption of the mineralized structures in the periradicular area (dentin, cementum, and bone tissue), manifested as radiolucent areas in radiographic images (8).
This inflammatory process is a host defense mechanism where synthesized cytokines trigger the immune response to control the infection. However, it simultaneously generates adverse effects such as pain, loss of bone support, or even tooth loss (10).
In both animal and human studies, radiographic imaging is commonly employed to ascertain the presence of PL and monitor its evolution following endodontic treatment (9,11-20).
In 1986, Orstarvik (21) introduced a scoring system for radiographic assessment of PL, simplifying Brynolf's proposed radiographic interpretation method. This system, known as the Periapical Index (PAI), uses an ordinal scale of 5 scores, ranging from 1 (healthy) to 5 (defined periapical bone destruction).
In 2010, the American Diabetes Association postulated that DM is a group of metabolic diseases characterized by hyperglycemia resulting from defects in insulin secretion, insulin action, or both (22).
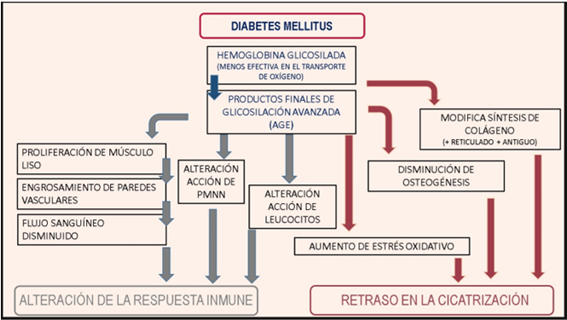
DM affects various functions of the immune system, altering cellular response and delaying healing (FIGURE 1). This situation leads to chronic inflammation and a predisposition to progressive tissue destruction (23).
The hyperglycemic state caused by DM affects circulation and induces various changes in pulp and periapical tissues (FIGURE 2). DM impairs the bactericidal function of neutrophils, severely affecting the immune response (24).
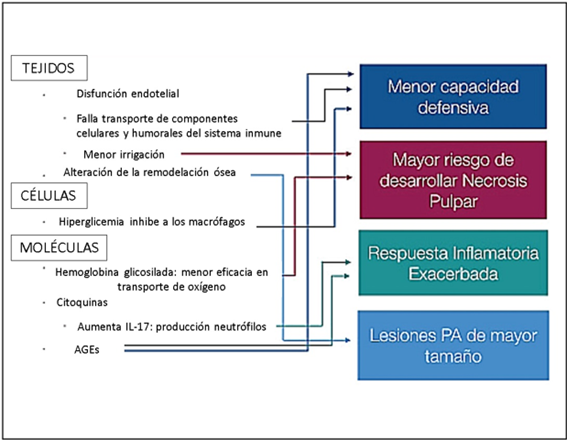
FIGURE 2: Diagram of the alterations produced by hyperglycemia and its possible endodontic consequences
According to a study published in 2020, patients with type 2 DM (primarily those who have had diabetes for several years) show an elevated Candida albicans count in primary infections of the canal system more frequently than healthy patients. This could explain these patients higher prevalence of periradicular processes (23).
This finding would allow us to propose a hypothesis regarding specific virulent endodontic pathogens present in the root canals of diabetic patients. It would also suggest that the high prevalence of these microorganisms may be one of the possible reasons why PL are more severe in diabetics than in non-diabetics (13,26).
Discussion
Several animal studies have demonstrated that the development and progression of apical periodontitis may be accelerated in the presence of diabetes. In comparison to control rats, diabetic rats exhibited increased inflammation of the periodontal ligament, heightened root resorption, and increased bone resorption. PL in diabetic rats were significantly larger than those in control rats, which suggests an intense inflammatory response under hyperglycemia conditions. (13,22,27-30).
The impact of DM on the prevalence and severity of PL was also assessed in humans through observational studies and clinical trials (9,15,19,20,31-35).
Segura-Egea et al. conducted a retrospective study at the Faculty of Dentistry of the University of Seville, where they found that type 2 diabetes is significantly associated with an increased prevalence of PL (35).
The radiographic prevalence of PL and endodontically treated teeth (ETT) was evaluated in patients with type 2 DM and healthy patients in a cross-sectional study conducted in a population of Catalonia, Spain (9). The results showed that, in adult patients, type 2 DM is significantly associated with a higher prevalence of PL and endodontic treatment.
In 2012, Marotta et al. conducted another cross-sectional study, considering the same parameters (PL and ETT prevalence in patients with type 2 DM), but this time in a Brazilian population (19). PL were significantly more prevalent in diabetic individuals compared to non-diabetic controls.
Another cross-sectional study, published in 2015, investigated the association between the prevalence of ETT and PL, as well as some systemic conditions or habits in an adult Portuguese population (15). A positive correlation between ETT and DM was observed, but the data were not statistically significant.
Some studies include aspects related to the evolution of DM or glycosylated hemoglobin (HbA1c) levels in their methodology (20,31-34).
A 2014 study investigated the frequency of periradicular lesions in long-term (> 48 months) and short-term (< 48 months) diabetic patients, showing a higher prevalence in patients with long-term DM (34).
In the same year, another cross-sectional study (20) analyzed radiographs of 83 patients with type 2 DM, applying the PAI. Patients were divided into two groups based on their disease control level: patients with well-controlled DM (HbA1c < 6.5%) and patients with poorly-controlled DM (HbA1c > 6.5%). The results of the final logistic regression model revealed a significantly higher prevalence of periradicular lesions in type 2 diabetic patients with HbA1c levels ≥ 6.5%, meaning poorly-controlled DM.
Computed tomography was used in a cross-sectional study assessing a potential association between type 2 DM and PL. A higher prevalence of PL and severe bone destruction was observed in patients with DM compared to non-diabetic patients. However, there were no significant differences between the subgroups with well-controlled and poorly-controlled DM (31).
Another finding emerged from a cross-sectional study published in 2020 where radiographs of 216 patients with type 2 diabetes were examined .The results showed no association between glycemic control and the prevalence of PL or endodontic treatment in type 2 diabetic patients.(32).
The results reported in these studies are inconclusive, but they reveal some differences in the natural history of PL in diabetic patients, suggesting an association between DM and PL (9).
The study of the severity and prevalence of PL in diabetic patients has been the focus of numerous literature reviews and meta-analyses. (4,8,10,25,36-44)
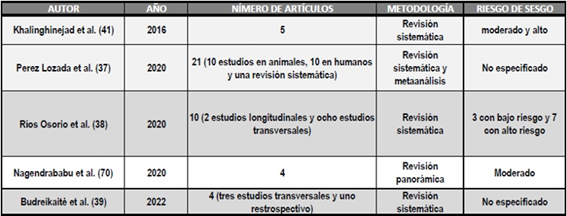
Table 1 outlines the methodological aspects of systematic reviews and meta-analyses.
TABLE 1: Systematic review articles and meta-analyses examining the prevalence and severity of PL in patients with DM.
According to some authors (39,41,43), current evidence is inconclusive and insufficient to suggest an association between DM and an increased prevalence and severity of PL.
Fouad (39) emphasizes that the available information regarding the pathogenesis, progression, and repair of PL in diabetic patients is still insufficient.
Several authors emphasize the importance of carefully considering diabetic patients (4, 38, 42, 43). They also highlight the risk of exacerbations and challenges in apical repair in these patients, emphasizing the necessity of understanding diabetic pathology to act consciously, safely, and effectively.
According to Lima (4), inadequate control of DM can predispose patients to oral infections, including dental pulp infection. Additionally, there is a correlation between the presence of apical periodontitis and patients with uncontrolled DM, indicating a cross-susceptibility between the two conditions.
Perez-Losada et al. (36) suggest, based on scientific evidence, a potential common pathophysiological factor between apical periodontitis and DM. However, further prospective studies are necessary to investigate the association between these two conditions.
Quezada(25) states that there is a higher prevalence of PL in diabetic patients and a statistically significant association between DM and the presence of PL in ETT, underscoring the importance of comprehensive patient care.
According to Cintra et al.(10) studies strongly suggest a bidirectional relationship between endodontic infection and diabetes.
Ríos-Osorio et al.(37) conclude that the results demonstrate biological foundations to suggest that type 2 DM may act as a risk factor for developing endodontic pathology.
Segura-Egea et al. from the University of Seville, Spain, have published reviews on the association between DM and PL (8, 40, 44). The authors state that the results of the studies are inconclusive but suggest an association between DM and PL. They assert that there is sufficient data in the literature to link DM to a higher prevalence of PL, larger size of osteolytic lesions, a greater likelihood of asymptomatic infections, and a worse prognosis for ETT. They also conclude that no scientific evidence demonstrates a causal effect of periapical inflammation on the metabolic control of diabetic patients(8). In 2019, they published a study aimed at analyzing the difference between association and causality, applying the causality criterion to the specific case of the association between endodontic disease and DM.
Budreikaitė et al.(38) found a statistically significant association between patients with type 2 diabetes and the presence of apical periodontitis, showing slowed post-treatment repair processes.
Although several studies demonstrate an association between the prevalence of PL, ETT, and lost ETT with systemic diseases such as DM, this does not prove the existence of a cause-effect relationship (44).
It is also essential to consider that most of the retrieved studies are cross-sectional, conditioning the analysis of the results, as the variables are obtained at the same time, and therefore, a cause-effect relationship cannot be established (33).
In the specific case of the association between PL and DM, cross-sectional studies lack information about the previous condition of the evaluated teeth, making it impossible to determine whether the images observed in paraclinical studies could potentially correspond to lesions in the healing process. The healing of periapical tissue is time-dependent and, therefore, challenging to evaluate through a cross-sectional study(27). This fact is particularly crucial in the context of this study since the higher prevalence of PL in diabetic patients could indicate a greater incidence of infection but also a delay in the repair process(44). Many studies utilize radiolucent images of the periapical region without considering whether the processes are in the repair or healing phase. This is significant because a paraclinical image might be classified as "endodontic failure" without knowledge of the pre-treatment diagnosis. Introducing the concept of "tolerance to endodontic treatment" could be valuable as a categorization for cases where certain aspects, such as the persistence of radiolucent images, make it inappropriate to include them in the "success" category, but the tooth remains functional and asymptomatic.
Controlling and evaluating all the variables involved in the outcome of endodontic treatment, which act as confounding factors, is often complex (31). Although the association between DM and periodontal disease is established, clinical studies do not assess probing depth nor consider periodontal disease as a cause of ETT loss in diabetic patients.
In the majority of studies, the determination of the diabetic patient group is based on anamnesis, without measuring glycemia or differentiating between type 1 and type 2 DM or well-controlled and poorly-controlled patients.
Nagendrababu (45) recommends conducting studies with representative and sufficient sample sizes, analyzing patients based on diabetes type, and determining their glycemic status through laboratory studies. Additionally, these studies should account for confounding factors such as periodontal disease, age, gender, examiner proficiency, quality of treatment, restorations, etc.
Conclusions
Studies linking DM to the prevalence and severity of PL, both in laboratory animals and in cross-sectional and longitudinal clinical studies in humans, remain contradictory and insufficient. However, the evidence indicates an association between DM and increased prevalence and severity of PL. This suggests that diabetic patients may experience apical periodontitis more frequently, with a more severe course and delayed repair following endodontic treatment (4,25,35,37,38).
Adequate follow-up of ETT and regular glycemic controls are essential to improve the endodontic prognosis (43). Due to the high prevalence of resistant biofilm detected in diabetic patients, professionals should be rigorous in controlling microorganisms such as C. albicans within the root canal system (23).
Diabetic patients require more rigorous follow-up, proactive interventional therapy rather than expectant approaches, communication with physicians, and increased preventive measures(46).
Since apical repair may be delayed after endodontic treatment, follow-up visits should be extended, if necessary, for more than four years (38).
REFERENCES
1. Holland R, Gomes Filho JE, Cintra LTA, Queiroz IODA, Estrela C. Factors affecting the periapical healing process of endodontically treated teeth. J Appl Oral Sci. 2017;25(5):465-76. [ Links ]
2. Ministerio de Salud Publica de Uruguay 2a Encuesta Nacional de Factores de Riesgo de Enfermedades No Transmisibles. 2013;83. Available from: https://www.gub.uy/ministerio-salud-publica/sites/ministerio-salud-publica/files/documentos/publicaciones/2DA_ENCUESTA_NACIONAL_final2_digital.pdf [ Links ]
3. Lorenzo-Erro SM, Skapino E, Musto M, Olmos P, Álvarez R, Fabruccini A, et al. Salud Bucal y Enfermedades no transmisibles en pacientes de un centro de enseñanza universitaria, Montevideo-Uruguay. Parte 1. Odontoestomatologia. 2020;22(36):55-64. [ Links ]
4. Lima SMF, Grisi DC, Kogawa EM, Franco OL, Peixoto VC, Gonçalves-Júnior JF, et al. Diabetes mellitus and inflammatory pulpal and periapical disease: A review. Int Endod J. 2013;46(8):700-9. [ Links ]
5. Tjäderhane L. Endodontic infections and systemic health - where should we go? Int Endod J. 2015;48(10):911-2. [ Links ]
6. Genco RJ, Borgnakke WS. Diabetes as a potential risk for periodontitis: association studies. Periodontol 2000. 2020;83(1):40-5. [ Links ]
7. Sanz M, Ceriello A, Buysschaert M, Chapple I, Demmer RT, Graziani F, et al. Scientific evidence on the links between periodontal diseases and diabetes: Consensus report and guidelines of the joint workshop on periodontal diseases and diabetes by the International Diabetes Federation and the European Federation of Periodontology. J Clin Periodontol. 2018;45(2):138-49. [ Links ]
8. Segura-Egea JJ, Martín-González J, Castellanos-Cosano L. Endodontic medicine: Connections between apical periodontitis and systemic diseases. Int Endod J. 2015;48(10):933-51. [ Links ]
9. López-López J, Jané-Salas E, Estrugo-Devesa A, Velasco-Ortega E, Martín-González J, Segura-Egea JJ. Periapical and endodontic status of type 2 diabetic patients in Catalonia, Spain: A cross-sectional study. J Endod. 2011;37(5):598-601. [ Links ]
10. Cintra LTA, Estrela C, Azuma MM, Queiroz índia O de A, Kawai T, Gomes-Filho JE. Endodontic medicine: Interrelationships among apical periodontitis, systemic disorders, and tissue responses of dental materials. Braz Oral Res. 2018;32:66-81. [ Links ]
11. Al-Zahrani MS, Abozor BM, Zawawi KH. The relationship between periapical lesions and the serum levels of glycosylated hemoglobin and C-reactive protein in type 2 diabetic patients. Saudi Med J. 2017;38(1):36-40. [ Links ]
12 Wolle CFB, Zollmann LA, Bairros PO, Etges A, Leite CE, Morrone FB, et al. Outcome of periapical lesions in a rat model of type 2 diabetes: Refractoriness to systemic antioxidant therapy. J Endod. 2013;39(5):643-7. [ Links ]
13. Armada-Dias L, Breda J, Provenzano JC, Breitenbach M, Rôças IDN, Gahyva SMM, et al. Development of periradicular lesions in normal and diabetic rats. J Appl Oral Sci. 2006;14(5):371-5. [ Links ]
14. Arya S, Duhan J, Tewari S, Sangwan P, Ghalaut V, Aggarwal S. Healing of Apical Periodontitis after Nonsurgical Treatment in Patients with Type 2 Diabetes. J Endod (Internet). 2017;43(10):1623-7. Available from: http://dx.doi.org/10.1016/j.joen.2017.05.013 [ Links ]
15. Correia-Sousa J, Madureira AR, Carvalho MF, Teles AM, Pina-Vaz I. Apical periodontitis and related risk factors: Cross-sectional study. Rev Port Estomatol Med Dent e Cir Maxilofac (Internet). 2015;56(4):226-32. Available from: http://dx.doi.org/10.1016/j.rpemd.2015.08.004 [ Links ]
16. Marques Ferreira M, Carrilho E, Carrilho F. Diabetes Mellitus e sua infuência no sucesso do tratamento endodôntico: Um estudo clínico retrospetivo. Acta Med Port. 2014;27(1):15-22. [ Links ]
17. Fouad, A. F. BJ. The effect of diabetes mellitus on endodontic treatment outcome Data from an electronic patient record. JADA. 2003;134(january):43-51. [ Links ]
18. Limeira FIR, Arantes DC, de Souza Oliveira C, de Melo DP, Magalhães CS, Bento PM. Root Canal Treatment and Apical Periodontitis in a Brazilian Population with Type 1 Diabetes Mellitus: A Cross-sectional Paired Study. J Endod. 2020;46(6):756-62. [ Links ]
19. Marotta PS, Fontes T V., Armada L, Lima KC, Rôças IN, Siqueira JF. Type 2 diabetes mellitus and the prevalence of apical periodontitis and endodontic treatment in an adult brazilian population. J Endod. 2012;38(3):297-300. [ Links ]
20. Sánchez-Domínguez B, López-López J, Jané-Salas E, Castellanos-Cosano L, Velasco-Ortega E, Segura-Egea JJ. Glycated Hemoglobin Levels and Prevalence of Apical Periodontitis in Type 2 Diabetic Patients. J Endod. 2015;41(5):601-6. [ Links ]
21. Orstavik D, Kerekes K, Eriksen HM. The periapical index: A scoring system for radiographic assessment of apical periodontitis. Dent Traumatol. 1986;2(1):20-34. [ Links ]
22. Cintra LTA, Samuel RO, Facundo ACS, Prieto AKC, Sumida DH, Bomfim SRM, et al. Relationships between oral infections and blood glucose concentrations or HbA1c levels in normal and diabetic rats. Int Endod J. 2014;47(3):228-37. [ Links ]
23. De la Torre-Luna R, Domínguez-Pérez RA, Guillén-Nepita AL, Ayala-Herrera JL, Martínez-Martínez RE, Romero-Ayala ME, et al. Prevalence of Candida albicans in primary endodontic infections associated with a higher frequency of apical periodontitis in type two diabetes mellitus patients. Eur J Clin Microbiol Infect Dis. 2020;39(1):131-8. [ Links ]
24. Sasaki H, Hirai K, M. Martins C, Furusho H, Battaglino R, Hashimoto K. Interrelationship Between Periapical Lesion and Systemic Metabolic Disorders. Curr Pharm Des. 2016;22(15):2204-15. [ Links ]
25. Quezada García MA, Palma Eyzaguirre AM. Relación bidireccional entre diabetes mellitus y periodontitis apical. ARS MEDICA Rev Ciencias Médicas. 2018;43(3):67-76. [ Links ]
26. Gomes CC, Guimarães LS, Pinto LCC, Camargo GADCG, Valente MIB, Sarquis MIDM. Investigations of the prevalence and virulence of candida albicans in periodontal and endodontic lesions in diabetic and normoglycemic patients. J Appl Oral Sci. 2017;25(3):274-81. [ Links ]
27. Iwama A, Nishigaki N, Nakamura K, Imaizumi I, Shibata N, Yamasaki M, et al. The effect of high sugar intake on the development of periradicular lesions in rats with type 2 diabetes. J Dent Res. 2003;82(4):322-5. [ Links ]
28. Kodama Y, Matsuura M, Sano T, Nakahara Y, Ozaki K, Narama I, et al. Diabetes enhances dental caries and apical periodontitis in caries-susceptible WBN/KobSlc rats. Comp Med. 2011;61(1):53-9. [ Links ]
29. Samuel RO, Ervolino E, de Azevedo Queiroz ÍO, Azuma MM, Ferreira GT, Cintra LTA. Th1/Th2/Th17/Treg Balance in Apical Periodontitis of Normoglycemic and Diabetic Rats. J Endod. 2019;45(8):1009-15. [ Links ]
30. Sano T, Matsuura T, Ozaki K, Narama I. Dental caries and Caries-Related periodontitis in type 2 diabetic mice. Vet Pathol. 2011;48(2):506-12. [ Links ]
31. Sisli SN. Evaluation of the Relationship between Type II Diabetes Mellitus and the Prevalence of Apical Periodontitis in Root-Filled Teeth Using Cone Beam Computed Tomography: An Observational Cross-Sectional Study. Med Princ Pract. 2019;28(6):533-8. [ Links ]
32. Pérez-Losada F, López-López J, Martín-González J, Jané-Salas E, Segura-Egea JJ, Estrugo-Devesa A. Apical periodontitis and glycemic control in type 2 diabetic patients: Cross-sectional study. J Clin Exp Dent. 2020;12(10):e964-71. [ Links ]
33. Smadi L. Apical periodontitis and endodontic treatment in patients with type II diabetes mellitus: Comparative cross-sectional survey. J Contemp Dent Pract. 2017;18(5):358-62. [ Links ]
34. Mesgarani A, Eshkevari N, Ehsani M, Khafri S, Nafarzade S, Damankesh Z. Frequency of odontogenic periradicular lesions in diabetic patients. Casp J Intern Med. 2014;5(1):22-5. [ Links ]
35. Segura-Egea JJ, Jiménez-Pinzón A, Ríos-Santos J V., Velasco-Ortega E, Cisneros-Cabello R, Poyato-Ferrera M. High prevalence of apical periodontitis amongst type 2 diabetic patients. Int Endod J. 2005;38(8):564-9. [ Links ]
36. Pérez-Losada F de L, Estrugo-Devesa A, Castellanos-Cosano L, Segura-Egea JJ, López-López J, Velasco-Ortega E. Apical Periodontitis and Diabetes Mellitus Type 2: A Systematic Review and Meta-Analysis. J Clin Med. 2020;9(2):540. [ Links ]
37. Riós-Osorio N, Munõz-Alvear HD, Canõ´n SM, Restrepo-Mendez S, Aguilera-Rojas SE, Jiménez-Penã O, et al. Association between type 2 diabetes mellitus and the evolution of endodontic pathology. Quintessence Int (Berl). 2020;51(2):100-7. [ Links ]
38. Budreikaitė K, Varoneckaitė M, Oleinikaitė D, Žilinskas J. Association between apical periodontitis and root canal treatment in patients with type II diabetes. A systematic review. Stomatologija. 2022;24(4):100-3. [ Links ]
39. Fouad AF. Diabetes Mellitus as a Modulating Factor of Endodontic Infections. J Dent Educ. 2003;67(4):459-67. [ Links ]
40. Segura-Egea JJ, Castellanos-Cosano L, Machuca G, López-López J, Martín-González J, Velasco-Ortega E, et al. Diabetes mellitus, periapical inflammation and endodontic treatment outcome. Med Oral Patol Oral Cir Bucal. 2012;17(2):356-61. [ Links ]
41. Khalighinejad N, Aminoshariae MR, Aminoshariae A, Kulild JC, Mickel A, Fouad AF. Association between Systemic Diseases and Apical Periodontitis. J Endod (Internet). 2016;42(10):1427-34. Available from: http://dx.doi.org/10.1016/j.joen.2016.07.007 [ Links ]
42. Tomazoli ATP, Endo MS, Pavan NNO. The implications of diabetes mellitus in Endodontics. Dent Press Endod. 2018;8(2):47-52. [ Links ]
43. Iliescu AA, Earar K, Iliescu MG, Ionel D, Gheorghiu IM, Iliescu A, et al. Chronic Apical Periodontitis and Diabetes Mellitus Relationship in Oral Rehabilitation. Rom J Oral Rehabil. 2019;11(1):71-8. [ Links ]
44. Segura-Egea JJ, Cabanillas-Balsera D, Jiménez-Sánchez MC, Martín-González J. Endodontics and diabetes: association versus causation. Int Endod J. 2019;52(6):790-802. [ Links ]
45. Nagendrababu V, Segura-Egea JJ, Fouad AF, Pulikkotil SJ, Dummer PMH. Association between diabetes and the outcome of root canal treatment in adults: an umbrella review. Int Endod J. 2020;53(4):455-66. [ Links ]
46. Ship JA. Diabetes and oral health: an overview. J Am Dent Assoc (Internet). 2003;134 Spec N:4S-10S. Available from: http://dx.doi.org/10.14219/jada.archive.2003.0367 [ Links ]
Conflict of Interest Statement: The authors have no conflict of interest in the publication of the article.
Authorship contribution note: 1.Study concept and design 2.Data acquisition 3.Data analysis 4.Discussion of results 5.Manuscript drafting 6.Approval of the final version of the manuscript MIG has contributed to: 1, 2, 3, 4, 5, 6 EA has contributed to: 1,4,5,6 AR has contributed: 1,4,5,6
Received: September 08, 2023; Accepted: November 03, 2023











 texto en
texto en