Servicios Personalizados
Revista
Articulo
Links relacionados
Compartir
Odontoestomatología
versión impresa ISSN 0797-0374versión On-line ISSN 1688-9339
Odontoestomatología vol.25 no.42 Montevideo 2023 Epub 01-Dic-2023
https://doi.org/10.22592/ode2023n42e230
Investigación
Effect of chlorhexidine pretreatment on demineralized dentin bond strength
1Cátedra de Materiales Dentales, Facultad de Odontología, Universidad de la República.
2 Facultad de Odontología. Universidad de la República.
3Cátedra de Odontopediatría y Servicio de Epidemiología y Estadística. Facultad de Odontología. Universidad de la República.
4Cátedra de Odontopediatría. Facultad de Odontología, Universidad de la República.
Objective:
To analyze the bond strength to healthy and demineralized dentin, immediately and after 6 months, using a 2% chlorhexidine (CHX) pretreatment.
Method
: 40 healthy third molars with incomplete root development were abraded exposing dentin. The pieces were subjected to pH cycling. They were randomly divided into 2 groups: with and without CHX. In dentin, 4 resin buttons were created using universal adhesive in self-etching mode. The samples were stored in distilled water at 37ºC until analysis. Micro shearing was carried out at 24 hours and at 6 months of aging.
Results:
Healthy dentin group, without immediate CHX presented higher bond strength (23.37±1.84). (Demineralized dentin group, without CHX, aged) presented the lowest bond strength (8.87±1.51).
Conclusions
: CHX prior to adhesive application doesn’t improve bond strength values to healthy or demineralized dentin in short nor long term.
Keywords: Demineralized dentin; Universal adhesives; Chlorhexidine; Young permanent teeth
Objetivo:
Analizar la resistencia de unión a dentina sana y desmineralizada, en forma inmediata y a los 6 meses, utilizando un pretratamiento de clorhexidina (CHX) 2%.
Método:
40 terceros molares sanos con desarrollo radicular incompleto se desgastaron exponiendo dentina. Las piezas fueron sometidas a ciclado de pH. Se dividieron aleatoriamente en 2 grupos: con y sin CHX. En dentina se crearon 4 botones de resina utilizando adhesivo universal mediante autoacondicionamiento. Las muestras se almacenaron en agua destilada a 37ºC hasta su análisis. El microcizallamiento se ejecutó a las 24 horas y a los 6 meses de envejecimiento.
Resultados:
El grupo de dentina sana, sin CHX inmediato presentó mayor resistencia adhesiva (23,37±1,84). El grupo de dentina desmineralizada, sin CHX, envejecido presentó la menor resistencia adhesiva (8,87±1,51).
Conclusiones:
La CHX al 2% previo a la aplicación del adhesivo no mejora los valores de resistencia de unión a dentina sana ni desmineralizada a corto o largo plazo.
Palabras clave: Dentina desmineralizada; Adhesivos universales; Clorhexidina; Dientes permanentes jóvenes
Objetivo:
Analisar a resistência de união à dentina hígida e desmineralizada, imediatamente e após 6 meses, utilizando um pré-tratamento com (CHX) a 2%.
Método:
40 terceiros molares hígidos com desenvolvimento radicular incompleto foram desgastados expondo a dentina. As peças foram submetidas a ciclagem de pH. Eles foram divididos aleatoriamente em 2 grupos: com e sem CHX. Em dentina, foram criados 4 botões de resina utilizando adesivo universal em modo autocondicionante. As amostras foram armazenadas em água destilada a 37ºC até a análise. O microcisalhamento foi realizado às 24 horas e aos 6 meses de envelhecimento.
Resultados:
O grupo de dentina saudável, sem CHX imediata apresentou maior resistência adesiva (23,37±1,84). O grupo de dentina desmineralizada, sem CHX , envelhecida apresentou a menor resistência adesiva (8,87±1,51).
Conclusões
: A CHX antes da aplicação do adesivo não melhoraria os valores de resistência de união em dentina saudável ou desmineralizada a curto ou longo prazo.
Palavras-chave: Dentina desmineralizada; Adesivos universais; Clorexidina; Dentes permanentes jovens
Introduction
Restorative dentistry has made progress in the selective removal of carious tissue (RSTC) in young adults. Currently, it is a recommended treatment for controlling the progression of carious lesions 1.
Schwendicke (2016) et al. 2 state that maintaining a reduced bacterial remnant in the cavity floor, deprived of nutrients, becomes irrelevant, as microorganisms sealed under a satisfactory restoration remain viable but inactive, preventing lesion progression. RSTC is recognized as a valid option for minimally invasive treatments that combine adhesive dentistry and restorative biomaterials. Current adhesive systems have not only improved their performance but also involve fewer handling steps, often offering advantages in bond strength to dentin substrates (3,4,5.
Adhesion to dentin poses a challenge beyond scientific advances, as its predominant organic component and moisture create an unfavorable terrain for hydrophobic adhesives. The hybrid layer formed between the adhesive system and dentin undergoes degradative processes of the resin and collagen components, as the monomers do not completely infiltrate the exposed collagen fibers 6,7. To overcome these challenges, universal adhesives have emerged, capable of bonding to various substrates such as enamel, dentin, metals, and ceramics 8,9. The main feature of universal adhesives is the inclusion of the 10-MDP molecule, a bifunctional acidic monomer (dehydrogenated methacryloyloxydecyl-phosphate) with the ability to bind to the calcium in hydroxyapatite, forming a less soluble and more stable salt - Ca-10MDP 10,11,12. Additionally, universal adhesives enable proper priming and interaction with dentin tissue, which is naturally wet; they exhibit a higher degree of polymerization, reducing residual free monomers and repelling water to prevent hydrolysis, thereby providing enhanced stability of the hybrid layer 5,11. Unprotected collagen fibers can be degraded by endogenous proteolytic enzymes found in dentin, known as matrix metalloproteinases (MMPs). CHX is an inhibitor of proteolytic activity. Scientific evidence shows 13,14 that CHX inhibits MMPs by its zinc and calcium chelating action preventing MMPs from performing their catalytic action. CHX at 2% has an extrinsic inhibitory effect on MMPs, especially MMP-2, MMP-8 and MMP-9 15. In this study, the use of 2% CHX within the adhesive protocol has been proposed as a way to prevent the degradation of the exposed collagen, thus delaying the degradation of the hybrid layer, which will be responsible for successful adhesion.
Objectives
To analyze the effect of a 2% CHX pretreatment on healthy and demineralized dentin through the bond strength of a universal adhesive, immediately and after 6 months.
Method
An in vitro, experimental, and longitudinal study was conducted at the Laboratory of Analysis and Development of Biomaterials (LADBio) at the Faculty of Dentistry (FO), University of the Republic (Udelar). The sample size was calculated using the SigmaPlot 12.0 program, considering a power of 80%, a type I error of 5%, and taking into account results reported in the literature 16. The calculation determined that a minimum of 8 specimens per group was needed to detect differences. The sample comprised 40 healthy, intact third molars that had not completed root development. These teeth were collected from the Surgical Block of the FO (all extractions were indicated for reasons beyond the scope of this research). Patients provided written consent for donation to the research.
Laboratory procedures
Once extracted, the teeth were stored in 0.5% Chloramine T for seven days and then in distilled water at a temperature of 3° to 5°C until the time of the study, for no more than three months. Each tooth was transversely abraded using a refrigerated trimmer, removing the enamel from the occlusal face, exposing a coronal dentin surface without pulp exposure. The abraded pieces were embedded in polypropylene (PPL) tubes using acrylic resin, leaving the dentin surface exposed. After being embedded, the exposed dentin surfaces were sequentially polished with silicon carbide sandpaper of 220, 400, and 600 grit size to standardize them (Fig. 1).
Once the standardization process was completed, the sample was randomly divided into two main groups of 20 molars each. One group was healthy dentin (DS) and the other group was demineralized dentin (DD). In the DS group, the smear layer was standardized by underwater sanding using 600g sandpaper. For the DD group, a protocol previously established in the literature was used. 17) The pieces included in this group were subjected to pH cycling. Initially, they were immersed in 10 mL of a demineralizing solution (2.2 mM CaCl2 + 2.2 mM KH2PO4 + 50 mM acetic acid at pH 4.8) for 8 hours. They were then immersed in a remineralizing solution (1.5 mM CaCl2 + 0.9 mM KH2PO4 + 0.15M KCl at pH 7) for 16 hours. This cycling was carried out for 14 days at room temperature and under agitation. Once this period was over, the samples were washed using distilled water. Finally, the DD surface was sanded with 600g sandpaper for 30 seconds creating a demineralized surface with smear.
Then, the specimens were randomly divided into two subgroups (10 pieces per group) according to the application or not of a 2% CHX pretreatment. In the CHX pretreatment subgroups, a 2% aqueous solution of CHX (Laboratorio Abarly S.A. Lot 67162. Reg. MSP 38840) was applied with a microbrush for 15 seconds. After removing the excess water, the Single Bond Universal adhesive system (3M ESPE, USA) was applied to all groups using the self-etching adhesive technique, strictly following the manufacturer's instructions. After the adhesive strategy was completed in all groups, a cylindrical silicone matrix with four holes of 1.4 mm internal diameter was placed on the dentin surface. Each of the holes was filled with composite resin (Z250xt, 3M ESPE, USA). The resin was handled according to the manufacturer's instructions: light-cured for 20 seconds using a light-curing unit (Optilight MAX, Gnatus, Brazil) with an intensity of 1000mW/cm2 which was previously tested with a radiometer (Bluelight Metter, Ivoclar Vivadent, Liechtenstein). Immediately after photopolymerization, the silicone matrix was removed to expose the four resin cylinders.
Once this process was completed, eight groups were created, which are described below; the first four groups correspond to healthy dentin (DS) and the remaining four to demineralized dentin (DD). Also, the 2% chlorhexidine pretreatment is expressed in each group and whether the group was subjected to the immediate test or after six months.
- Group 1: Healthy dentin, with 2% chlorhexidine pretreatment, immediate shear test.
- Group 2: Healthy dentin, with 2% chlorhexidine pretreatment, shear test after six months.
- Group 3: Healthy dentin, without 2% chlorhexidine pretreatment, immediate shear test.
- Group 4: Healthy dentin, without 2% chlorhexidine pretreatment, shear test after six months.
- Group 5: Demineralized dentin, with 2% chlorhexidine pretreatment, immediate shear test.
- Group 6: Demineralized dentin, with 2% chlorhexidine pretreatment, shear test after six months.
- Group 7: Demineralized dentin, without 2% chlorhexidine pretreatment, immediate shear test.
- Group 8: Demineralized dentin, without 2% chlorhexidine pretreatment, shear test after six months.
All specimens were immersed in distilled water at 37°C for 24 hours. Subsequently, two out of the four resin cylinders from each specimen underwent the micro-shear test. After the initial testing, the specimens with the remaining two resin cylinders were stored in distilled water at 37°C for six months. Following the aging period, the remaining two cylinders in each specimen underwent the micro-shear test. Shear bond strength testing was conducted in accordance with ISO 29022 (18) using a universal mechanical testing machine (CMT 2000, MTS SANS, China). A 0.5 mm diameter stainless steel wire loop was precisely positioned at the resin-dentin adhesive interface at a crosshead speed of 1.0 mm/min (Fig. 2). The bond strength (in MPa) was calculated by dividing the load (in Newtons) by the bond interface area (mm2).
Statistical analysis
The bond strength values are reported descriptively by averages and standard deviation in each group. Comparisons were made by means of a mixed ANOVA model, taking the following as fixed factors: pretreatment (with and without CHX), time (immediate and after 6 months) and dentin condition (DS and DD). Meanwhile, the intra-individual variation of each tooth was considered as a random factor. A statistical significance of 5% was established for all tests. All analyses were performed with R software for Windows (R Core Team, 2013. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL http://www.R-project.org/).
Results
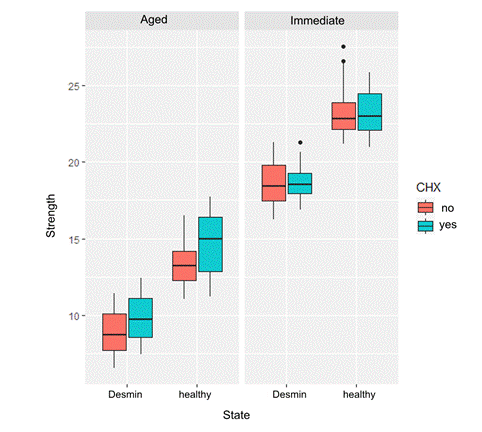
The average values obtained during the micro-shear test (MPa) are shown in Fig. 3. It can be seen that group 3 (DS, without immediate CHX pretreatment) had the highest average bond strength values (23.37±1.84), while group 8 (DD, without aged CHX pretreatment) had the lowest average bond strength values (8.87±1.51).
The variations in bond strength values among different specimens were explained through a marginal effects model, taking into account three factors: pretreatment (with and without CHX), time (immediate and six months), and dentin status (DS and DD) (Table 1). A significant interaction of pretreatment with CHX over time (immediate and six months) in bond strength values was observed with a likelihood ratio test LRT=33.93, p≤0.001. However, there was no significant interaction of CHX pretreatment concerning dentin status (DS and DD) on bond strength, as shown by LRT=0.02, p=0.88.
| Model | df | LRT | p-value |
|---|---|---|---|
| Marginal Effects | 6 | ||
| + CHX Interaction: Time | 7 | 33,93 | <0,001 |
| + CHX Interaction: State | 8 | 0,02 | 0,882 |
Table 1. Interaction between CHX and time/state.
A significant effect of CHX pretreatment was observed concerning time (p < 0.001), but not regarding the demineralization state (p = 0.882). Multiple comparisons (Table 2) indicated that bond strength in the immediate test between CHX-pretreated cylinders did not significantly differ from untreated ones (p = 0.89). However, after six months, a statistically significant difference of 1.2 MPa was observed between the CHX-treated group and the non-CHX group (p ≤ 0.001). Both comparisons were adjusted based on their demineralization state.
| Estimation | Standard deviation | p-value | |
|---|---|---|---|
| Immediate | |||
| with CHX | 21,0 | 0,34 | 0,89 |
| without CHX | 21,0 | 0,34 | |
| Aged | |||
| with CHX | 12,3 | 0,34 | <0,001 |
| without CHX | 11,1 | 0,34 |
Table 2. Multiple comparisons
Fig. 4 shows that the distribution of bond strength values did not differ when comparing the immediate CHX-treated and untreated cylinders. However, after six months, the bond strength decreased compared to the initial levels. The demineralized samples exhibited lower bond strength (irrespective of pretreatment). As indicated in Table 2, at six months, there appears to be a slightly higher bond strength in the CHX-pretreated cylinders (irrespective of the demineralization state).
Discussion
In our study, we were able to confirm that 2% CHX prior to adhesive application does not improve bond strength values to healthy or demineralized dentin neither in the short nor long term. Bond strength values did not show immediate differences when comparing groups with or without CHX pretreatment. At six months, a slightly higher bond strength seems to be evident in the CHX-pretreated cylinders (irrespective of the dentin state).
Regarding the generation of demineralized dentin in the laboratory, scientific evidence has shown that there are different protocols to achieve this. Marquezan et al. 17) proposed a protocol with pH cycling as they considered it more appropriate to simulate a substrate resembling the dentin layer affected by caries.
Moreover, Koyuturk et al. 19 reported that the primary cause of reduced bond strength in carious human dentin could be attributed to the components of the biomaterials used, rather than the acidity of the monomers included in self-adhesive systems. Additionally, Hosoya et al. 20) proposed that the altered mineral in the interfibrillar space of demineralized dentin might influence the formation of the hybrid layer and the chemical bonding with carboxylic and phosphate derivatives of methacrylates. Shen et al. 21 concluded that the 10-MDP monomer reduces both MMP activation and nanofiltration through a mechanism involving the formation of Ca-MDP salts. While CHX may interfere with the formation of these salts when applied in conjunction with 10-MDP, it does not have a detrimental effect. Furthermore, the bonding performance is enhanced by the application of 10-MDP.
As indicated in the literature, adhesives containing 10-MDP exhibit prolonged adhesion 22. Lima (23) demonstrated the presence of MMPs beneath the hybrid layer of exposed and non-infiltrated collagen. These enzymes can be activated by the presence of weak acids in adhesive systems. High dentin bond strength was achieved when the adhesive effectively infiltrated acid-exposed collagen or inhibited MMPs in the demineralized zone, responsible for degrading proteins like collagen and elastin (24.
In summary, while there is evidence suggesting that CHX can inhibit MMP activity, its impact on bond strength in universal adhesive systems remains unclear due to contradictory results23.
Regarding adhesive strategies, the use of CHX as an antiseptic before adhesive application does not directly impact the bond strength to demineralized dentin. Conversely, according to de Breschi et al. 25, the use of CHX has a direct influence on inhibiting the MMPs present in dentin. In line with the findings of Breschi et al. 25, the results from the study by Tessore R et al. 26, which examined the adhesive efficacy of two universal adhesive systems-one of them containing CHX in its composition-demonstrated that the use of CHX does not influence bond strength to dentin. Although MMPs' ability to degrade the extracellular matrix was recognized decades ago, the correlation between nanofiltration and hybrid layer degradation was not established until 1999 when Sano 27 and his research team demonstrated the hydrolytic degradation of collagen in the hybrid layer. The water within the hybrid layer serves as a functional medium for the hydrolysis of the resin matrix. This adhesive hydrolysis is considered the main reason for the degradation of the hybrid layer, consequently affecting bond strength over time 28.
In the systematic review and meta-analysis conducted by Kiuru et al. 29, a total of 43 articles were analyzed, and 21 articles involving CHX treatments were included for meta-analysis. The results clearly demonstrate the benefits of inhibiting collagen-degrading enzymes in preserving dentin bond strength. Given that CHX exhibits no adverse effects on immediate bond strength, its clinical use to enhance the longevity of resin-dentin bonds can be recommended.
One limitation of this study is that the demineralized dentin achieved in the laboratory may not precisely replicate human carious dentin. However, it can be considered highly analogous, especially for the evaluation of pretreatment. It is important to acknowledge that, in clinical application, certain biases might influence the results.
REFERENCES
1. Casagrande L, Seminario AT, Correa MB, Werle SB, Maltz M, Demarco FF, et al. Longevity and associated risk factors in adhesive restorations of young permanent teeth after complete and selective caries removal: a retrospective study. Clin Oral Investig. 2017 Apr;21(3):847-55. https://doi.org/10.1007/s00784-016-1832-1 [ Links ]
2. Schwendicke F, Frencken JE, Bjørndal L, et al. Managing Carious Lesions: Consensus Recommendations on Carious Tissue Removal.Advances in Dental Research. 2016;28(2):58-67. doi:10.1177/0022034516639271 [ Links ]
3. Uribe S. Partial caries removal in symptomless teeth reduces the risk of pulp exposure. Evidence-Based Dentistry. 2006 Dec 24;7(4):94-94. [ Links ]
4. Li T, Zhai X, Song F, Zhu H. Selective versus non-selective removal for dental caries: a systematic review and meta-analysis. Acta Odontologica Scandinavica. 2018 Feb 17;76(2):135-40. [ Links ]
5. Nagarkar S, Theis‐Mahon N, Perdigão J. Universal dental adhesives: Current status, laboratory testing, and clinical performance. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2019 Aug 14;107(6):2121-31. [ Links ]
6. Rodrigues JA, Casagrande L, Araújo FB, Lenzi TL, Mariath AAS. Restorative Materials in Pediatric Dentistry. In: Pediatric Restorative Dentistry. Cham: Springer International Publishing; 2019. p. 161-7. [ Links ]
7. Banerjee A. Minimal intervention dentistry: part 7. Minimally invasive operative caries management: rationale and techniques. British Dental Journal. 2013 Feb 8;214(3):107-11. [ Links ]
8. Rosa WL de O da, Piva E, Silva AF da. Bond strength of universal adhesives: A systematic review and meta-analysis. Journal of Dentistry. 2015 Jul 1;43(7):765-76. [ Links ]
9. Perdigão J, Swift EJ.. Universal Adhesives. Journal of Esthetic and Restorative Dentistry. 2015 Nov 1;27(6):331-4. [ Links ]
10. Elkaffas AliA, Hamama HHH, Mahmoud SH. Do universal adhesives promote bonding to dentin? A systematic review and meta-analysis. Restorative Dentistry & Endodontics. 2018;43(3). [ Links ]
11. Shadman N, Farzin-Ebrahimi S, Mortazavi-Lahijani E, Jalali Z. Effect of chlorhexidine on the durability of a new universal adhesive system. Journal of Clinical and Experimental Dentistry. 2018;10(9):0-0. [ Links ]
12. Chen C, Niu L-N, Xie H, Zhang Z-Y, Zhou L-Q, Jiao K, et al. Bonding of universal adhesives to dentine - Old wine in new bottles? Journal of Dentistry. 2015 May 1;43(5):525-36. [ Links ]
13. Osorio R, Yamauti M, Osorio E, Ruiz-Requena ME, Pashley D, Tay F, et al. Effect of dentin etching and chlorhexidine application on metalloproteinase-mediated collagen degradation. European Journal of Oral Sciences. 2011 Feb;119(1):79-85. [ Links ]
14. Bravo C, Sampaio CS, Hirata R, Puppin-Rontani RM, Mayoral JR, Giner L. In-vitro Comparative Study of the use of 2 % Chlorhexidine on Microtensile Bond Strength of Different Dentin Adhesives: A 6 Months Evaluation. International Journal of Morphology. 2017 Sep;35(3):893-900. [ Links ]
15. Mazzoni A, Nascimento FD, Carrilho M, Tersariol I, Papa V, Tjäderhane L, et al. MMP Activity in the Hybrid Layer Detected with in situ Zymography. Journal of Dental Research. 2012 May 21;91(5):467-72. [ Links ]
16. Mobarak EH, El-Korashy DI, Pashley DH. Effect of chlorhexidine concentrations on micro-shear bond strength of self-etch adhesive to normal and caries-affected dentin. American journal of dentistry. 2010;23(4):217-22. [ Links ]
17. Marquezan M, Corrêa FNP, Sanabe ME, Rodrigues Filho LE, Hebling J, Guedes-Pinto AC, et al. Artificial methods of dentine caries induction: A hardness and morphological comparative study. Archives of Oral Biology. 2009 Dec;54(12):1111-7. [ Links ]
18. International Organization for Standarization. ISO 29022:2013 - Dentistry - Adhesion - Notched-edge shear bond strength test. 2013https://www.iso.org/standard/45285.html [ Links ]
19. Koyuturk A, Sengun A, Ozer F, Sener Y, Gokalp A. Shear Bond Strengths of Self-etching Adhesives to Caries-affected Dentin on the Gingival Wall. Dental Materials Journal. 2006;25(1):59-65. [ Links ]
20. Hosoya Y, Tay FR, Ono T, Miyazaki M. Hardness, elasticity and ultrastructure of primary tooth dentin bonded with a self-reinforcing one-step self-etch adhesive. Journal of Dentistry. 2010 Mar;38(3):214-21. [ Links ]
21. Shen J, Xie H, Wang Q, Wu X, Yang J, Chen C. Evaluation of the interaction of chlorhexidine and MDP and its effects on the durability of dentin bonding. Dental Materials. 2020 Dec 1;36(12):1624-34. [ Links ]
22. Bahari M, Oskoee SS, Esmaeel M, Chaharom E, Kahnamoui MA, Gholizadeh S, et al. Effect of accelerated aging and double application on the dentin bond strength of universal adhesive system. Dental Research Journal. 2021;1. [ Links ]
23. Lima JFM de, Wajngarten D, Islam F, Clifford J, Botta AC. Effect of adhesive mode and chlorhexidine on microtensile strength of universal bonding agent to sound and caries-affected dentins. European Journal of Dentistry. 2018 Oct 23;12(04):553-8. [ Links ]
24. Singh D, Srivastava SK, Chaudhuri TK, Upadhyay G. Multifaceted role of matrix metalloproteinases (MMPs). Frontiers in Molecular Biosciences. 2015 May 13;2(MAY):19. [ Links ]
25. Breschi L, Mazzoni A, Nato F, Carrilho M, Visintini E, Tjäderhane L, et al. Chlorhexidine stabilizes the adhesive interface: A 2-year in vitro study. Dental Materials. 2010 Apr;26(4):320-5. [ Links ]
26. Tessore R, Silveira C, Vázquez P, Mederos M, García A, Cuevas-Suarez CE, et al. Evaluación de la resistencia de unión a dentina humana de un sistema adhesivo universal con clorhexidina utilizado en modo de grabado total y autocondicionante. Odontoestomatología. 2020 Jun 6;22(35):20-9. [ Links ]
27. Sano H, Yoshikawa T, Pereira RNR, Kanemura N, Morigami M, Tagami J, et al. Long-term durability of dentin bonds made with a self-etching primer, in vivo. Journal of dental research. 1999;78(4):906-11. [ Links ]
28. Frassetto A, Breschi L, Turco G, Marchesi G, di Lenarda R, Tay FR, et al. Mechanisms of degradation of the hybrid layer in adhesive dentistry and therapeutic agents to improve bond durability--A literature review. Dental materials: official publication of the Academy of Dental Materials. 2016 Feb 1;32(2):e41-53. [ Links ]
29. Kiuru O, Sinervo J, Vähänikkilä H, Anttonen V, Tjäderhane L. MMP Inhibitors and Dentin Bonding: Systematic Review and Meta-Analysis. Isola G, editor. International Journal of Dentistry. 2021 May 27;2021:1-14. [ Links ]
Conflict of Interest Statement: The authors have no conflict of interest in the publication of the article.
Authorship contribution note: Study concept and design Data acquisition Data analysis Discussion of results Manuscript drafting Approval of the final version of the manuscript AG has contributed in 1, 2, 3, 4, 5, and 6. MCLJ has contributed in: 1, 3, 4, 5 and 6 AF has contributed in 3, 6 JL has contributed in: 1, 3, 4, 5 and 6
Received: May 26, 2023; Accepted: September 12, 2023











 texto en
texto en