Serviços Personalizados
Journal
Artigo
Links relacionados
Compartilhar
Odontoestomatología
versão impressa ISSN 0797-0374versão On-line ISSN 1688-9339
Odontoestomatología vol.24 no.40 Montevideo dez. 2022 Epub 01-Dez-2022
https://doi.org/10.22592/ode2022n40e317
Update
Antimicrobial effectiveness of chitosan as a suture coating in oral and maxillofacial surgery: a systematic review
1Universidad Nacional Autónoma de México, UNAM, México
2Laboratorio de Investigación Interdisciplinaria, Nanoestructuras y Biomateriales, Escuela Nacional de Estudios Superiores, Unidad León, Universidad Nacional Autónoma de México, León Guanajuato. México. carenas@enes.unam.mx
Postoperative infections are increasingly common, as well as the increase in bacterial resistance mechanisms to a diverse class of broad-spectrum antibiotics. Hence the need to find a biocompatible and antimicrobial natural material to use as suture coating since this is considered an intrinsic factor in the process of these complications. This review aims to carry out a systematic search on the antimicrobial effectiveness of chitosan used as suture coating in oral and maxillofacial surgery. The search was carried out in two databases: PubMed and ScienceDirect from February to March 2021. PRISMA guidelines for systematic reviews were followed. The five articles from in vitro or in vivo studies showed that chitosan coating on sutures inhibits Gram-positive and Gram-negative microorganisms, exhibiting greater activity against Gram-negative microorganisms. It is concluded that chitosan coating on sutures could supplement and improve the properties of currently commercial antimicrobial sutures, reduce postoperative infections, and accelerate the healing processes.
Keywords: chitosan; antimicrobial effect; oral surgery; sutures
Las infecciones postoperatorias son cada vez más comunes, así como el incremento en los mecanismos de resistencia bacteriana hacia una diversa clase de antibióticos de amplio espectro, por lo que actualmente ha surgido la necesidad de buscar materiales de origen natural con propiedades antimicrobianas y biocompatibles, que puedan evitar diversas complicaciones relacionadas con las infecciones postoperatorias. El objetivo de esta revisión sistemática es poner en contexto actual la efectividad antimicrobiana del quitosano empleado en el recubrimiento de suturas en cirugía oral y maxilofacial. La estrategia de búsqueda se llevó a cabo en dos bases de datos: PubMed y ScienceDirect de febrero a 18 de octubre del 2021. Se siguieron los lineamientos PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses). De acuerdo con nuestros resultados, los cinco artículos incluidos de estudios in vitro e in vivo, mostraron que el recubrimiento de suturas con quitosano inhibe a los microorganismos de tipo Gram (+) y Gram (-), presentando una mayor actividad contra la especie Gram (-). Se concluye, además, que el recubrimiento de suturas con quitosano podría suplir y mejorar las propiedades de suturas antimicrobianas actualmente comerciales, así como reducir las infecciones postoperatorias y acelerar los procesos de cicatrización.
Palabras clave: Quitosano; efecto antimicrobiano; cirugía oral; suturas
As infecções pós-operatórias são cada vez mais comuns, assim como o aumento dos mecanismos de resistência bacteriana a uma diversa classe de antibióticos de longo espectro, razão pela qual surgiu agora a necessidade de procurar materiais de origem natural com propriedades antimicrobianas e biocompatíveis, que pode prevenir várias complicações relacionadas a infecções pós-operatórias. O objetivo desta revisão sistemática é contextualizar a eficácia antimicrobiana da quitosana utilizada no revestimento de suturas em cirurgia bucomaxilofacial. A estratégia de busca foi realizada em dois bases de dados: PubMed e ScienceDirect de fevereiro a 18 de outubro de 2021. Foram seguidas as diretrizes PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses). De acordo com nossos resultados, os cinco artigos incluídos de estudos in vitro e in vivo mostraram que o revestimento das suturas com quitosana inibe microrganismos do tipo Gram (+) e Gram (-), apresentando maior atividade contra espécies Gram. (-). Conclui-se também que o revestimento das suturas com quitosana pode substituir e melhorar as propriedades das suturas antimicrobianas atualmente comerciais, além de reduzir infecções pós-operatórias e acelerar os processos de cicatrização.
Palavras-chave: Quitosana; efeito antimicrobiano; cirurgia oral; suturas
1. Introduction
Chitosan is a natural biopolymer consisting of D-glucosamine and N-acetyl-D-glucosamine units derived from the deacetylation of chitin, which is extracted from marine shell waste and insect exoskeletons with a coating structure.1
Chitosan has a lower molecular weight and crystallinity than chitin. It has unique properties such as compatibility, biodegradability, non-toxicity, mucoadhesion, biological activity against a wide range of bacteria and fungi, bioactive, analgesic, hemostatic, anti-erosive, anti-abrasive, and antioxidant properties.2 Depending on the use intended, chitosan can be modified through chemical or enzymatic processes to form various derivatives, such as gels, micro/nanoparticles, fibers, sponges, and films.3 Chitosan must have a higher degree of deacetylation when used to manufacture materials that require greater cell adhesion and proliferation since it helps activate fibroblasts and accelerate wound healing.4
Furthermore, oral and maxillofacial surgery is the medical-surgical specialty that deals with the prevention, diagnosis, treatment, and rehabilitation of pathologies of the face, cervical structures, and oral cavity. However, there may be high-risk immediate or postoperative complications. Immediate complications are intraoperative and affect dental organs, soft tissues, and hard tissues of the oral cavity. Postoperative complications occur 48 hours to 72 hours after surgery.5 The risk depends on several factors, such as surgery time, the type of wound and/or intervention, and the patient’s condition.6
The suture is a vital part of almost every surgical procedure. It is used to bind the skin surface and for vessel ligation. It is designed to close and stabilize wound margins and to allow for healing.7,8 The materials used to manufacture sutures must comply with specific biological characteristics such as bacterial inhibition, tissue reaction, histocompatibility and resorption, and physical, mechanical, and handling characteristics (knot tensile strength, elasticity, caliber, capillarity, and surface).9 Therefore, suture materials can be considered intrinsic category risk factors that inhibit surgical wound healing.10
In dentistry, chitosan-based drugs are used to treat periodontal diseases, dental caries, root canal treatment (endodontics), and to provide prolonged local anesthetic action.11 Randomized clinical trials and in vitro or in vivo studies suggest that coating sutures with triclosan or natural extracts may help prevent surgical site infections.12 However, it has been shown that triclosan coating can negatively affect immune functions and cell reproducibility.13 Therefore, there is a growing need for a natural coating with a wide range of beneficial properties for the oral cavity, such as chitosan.14
This review aims to determine the antimicrobial effectiveness of chitosan used as silk suture coating in oral and maxillofacial surgery.
2. Methodology
The PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement criteria were considered for this systematic review.15
Our research question is: What is the antimicrobial effectiveness chitosan-coated sutures in oral and maxillofacial surgery?
PICOS Acronym: P=Post-surgical infections due to bacterial growth on sutures; I=Chitosan-coated suture, C=Sutures with and without chitosan, O=Effectiveness in reducing microbial growth rate, S=In vitro and in vivo studies.
2.1 Data sources and search strategy
The search was conducted from 20 February to 18 October, 2021, in PubMed and ScienceDirect using the keywords “chitosan,” “sutures,” and “antimicrobial effect.” The keywords were combined with the Boolean operators AND or OR in both databases.
2.2 Inclusion and exclusion criteria
The following articles were included: all publications from 2015 to 2021, full-text articles in English or Spanish, in vivo and in vitro studies evaluating chitosan-coated sutures with various pathogens (Staphylococcus aureus and Staphylococcus epidermidis as Gram-positive, Escherichia coli (Gram-negative) and Candida albicans as a fungal pathogen.
The studies that did not use chitosan for suture coating in oral and maxillofacial surgery were excluded, as well as mini-reviews and systematic reviews with or without meta-analysis.
2.3 Data selection, extraction, and analysis
PM-KDG and OU-MA analyzed the articles by title and abstract based on the inclusion criteria. They also extracted the following data: type of suture used, degree of chitosan deacetylation, chitosan concentration, additional agents or compounds, techniques or methods to incorporate the chitosan to sutures and their antimicrobial effectiveness by observing the variable response of minimum inhibitory concentration (MIC), zone of inhibition (ZI), and bacterial growth rate (GR).
2.4 Methodological quality and risk of bias
Quality analysis was conducted. It was performed a critical and hierarchical analysis, classifying the evidence available in the literature as high, moderate, low, and very low. Specific parameters were applied according to the presence of the following elements: a comparison between two formulations, a control group, coating methods described in full, a statistical analysis, concordance in the measurement methods, and the results of the response variable.
3. Results
3.1 Study selection
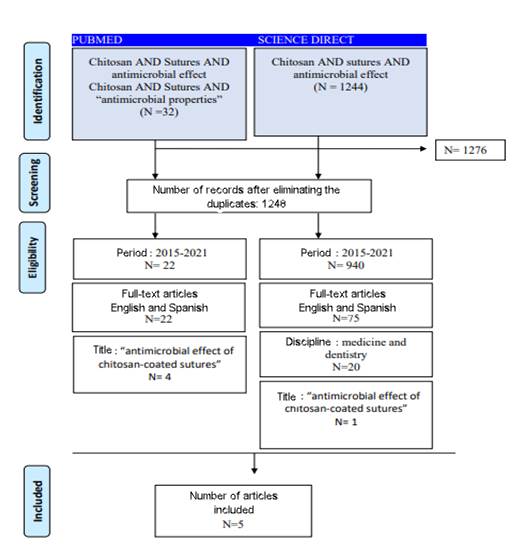
The PRISMA diagram in Figure 1 shows the results obtained through the search parameters established above.
The initial search yielded 1275 articles. After eliminating duplicate articles, 1248 articles remained. Then, we considered the 5-year publication period and the full-text articles, and 22 articles were obtained. These were reviewed based on the inclusion criteria. A total of 5 articles resulted from this process. They were analyzed as presented in this systematic review.
Coating the sutures with antimicrobial agents or antibiofilm would prevent microorganism adhesion. In turn, this would restrict biofilm formation and inhibit infection. Natural coating formulations effectively reduce the use of topical and toxic suture treatments. Results indicate that applying natural coating materials over non-absorbable sutures may help reduce the number of incisions and wound-site infections.17 Including other materials in this coating could improve the suture’s physical and biological properties. Table 1 shows the results of the antimicrobial effect of chitosan-coated sutures.
Masood et al.16 evaluated the in vitro antimicrobial effectiveness of the coating of two types of suture: polyethylene terephthalate (PET) and polyamide (Nylon 6) multifilament threads, adding chitosan (0.75% w/v) with a deacetylation degree of 90-95%, starch (1.5% w/v), softener (0.2% w/v), clove oil (0.05% w/v), turmeric oil (0.5% w/v) in different combinations. All these coating formulations have shown satisfactory antimicrobial activity against Gram-positive microorganisms.
Subramani Prabha et al.17 conducted an in vitro study to evaluate the antimicrobial effectiveness of two types of chitosan (extracted from crab shells (EC) and commercial (CC)) as a coating on braided vicryl absorbable sutures against Gram-positive S. epidermidis and Gram-negative C. albicans. The results indicate that the uncoated suture (control) and sutures soaked with 1% (v/v) acetic acid (vehicle control) did not show any zone of clearance, indicating a lack of antibacterial or anti-candidal activity. Both EC (200 µg/ml) and CC (200 µg/ml) impregnated sutures showed antibacterial and antifungal activity. EC-impregnated sutures showed a clear zone of inhibition for both test strains, which seems evident given their more effective antimicrobial activity. In turn, CC-impregnated sutures showed a minimal zone of inhibition for both test strains.
Faten Debbabi et al.18 evaluated the antimicrobial effectiveness of 16-filament PA 6-6 braided sutures coated with chitosan (1%, 2%, and 3%) with a deacetylation degree higher than 75%, citric acid (3%, 6%, and 10%) and sodium hypophosphite (1%, 2%, and 3.5 %) against Gram-positive S. aureus, Gram-positive S. epidermidis, Gram-negative E. coli, and Gram-negative P. aeruginosa. The results indicate that the uncoated suture did not inhibit the growth of bacteria, which expanded completely around it. On the other hand, the sutures coated with 1% and 2% chitosan showed greater inhibition for the four types of bacteria, showing that the zone of inhibition (ZI) decreased as the chitosan concentration increased.
Hassan Mohammadi et al.19 evaluated the coating of a monofilament Nylon suture by adding hyaluronic acid (HA) and chitosan at different concentrations and a 75-85% degree of deacetylation. The chitosan (4% w/v) and chitosan (4% w/v)-hyaluronic acid (HA) (8% w/v) samples exhibited significant antibacterial activity against E. coli compared to the HA sample. This may be connected to the intrinsic antibacterial property of chitosan attributed to its positive charge, which induces strong reactions between the chitosan molecule and the negatively charged bacterial cell surface. In turn, chitosan (4% w/v) shows no antibacterial activity against S. aureus, probably due to the low chitosan concentration. However, adding HA as a second layer generates antibacterial activity that lasts up to 70 hours.
Ying Yang et al.20 conducted an in vitro and in vivo study of antibacterial absorbable sutures (VP) and absorbable sutures (V) gentamicin-coated Vicryl (GV) suture, and hydroxypropyltrimethyl ammonium chloride chitosan (HACC)-coated Vicryl (HV) suture at an 87% concentration. The zone of inhibition of the three antibiotic-coated sutures (VP, GV, and HV) exhibited no significant difference for S. epidermidis (p>0.05). In contrast, the zone of inhibition of GV against S. aureus was evidently smaller than that of VP and HV (p<0.01).
Table 1: Summary of individual articles
| Author/ Year | Coating materials | Chitosan concentration | Chitosan degree of deacetylation | Coatings | Type of suture used | Type of bacteria | Antimicrobial effectiveness |
| Ying Yang et al., 2016 | Hydroxypropyl trimethyl ammonium chloride chitosan Gentamicin sulfate salt | Mass concentration of 0.2 wt.% dissolved in 50 mL of collagen solutions | 87% | 1. Gentamicin-coated Vicryl (GV) suture 2. Hydroxypropyltrimethyl ammonium chloride chitosan (HACC)-coated Vicryl (HV) suture 3. (V) absorbable Vicryl suture 4. (VP) Vicryl Plus | Vicryl absorbable suture | Staphylococcus epidermidis (Gram-positive) Staphylococcus aureus (Gram-positive) | POSITIVE (ZI)=The three sutures VP, GV and HV, showed no significant difference for S. epidermidis (p>0.05), while the zone of inhibition of GV against S. aureus was clearly smaller than that of VP and HV (p<0.01). |
| Faten Debbabi et al., 2017 | Chitosan with citric acid and sodium hypophosphite | Variable concentrations for chitosan (1%, 2%, and 3%) and citric acid | Higher than 75% | 9 coatings considering 1. Chitosan concentration (1%, 2%, and 3%). 2. Citric acid concentration (3%, 6%, and 10%) 3. Sodium hypophosphite concentration (1%, 2%, and 3.5%) | PA 6-6 braided suture-16 filaments | Staphylococcus aureus (Gram-positive) Staphylococcus epidermidis (Gram-positive) Escherichia coli (Gram-negative) Pseudomona aeruginosa (Gram-negative) | POSITIVE higher (ZI): (ZI=9.5 ± 0.7) mm against P. aeruginosa, 1% chitosan concentration. (ZI=3.75 ± 0.3) mm against S. epidermis, 2% chitosan. (ZI=5.75 ± 0.3) mm against S. aureus, 1% chitosan. (ZI=8.75 ± 0.3) mm against E. coli, 1% chitosan. |
| Masood et al., 2017 | Starch Softener Clove oil Turmeric Hydrolysed chitosan | 0.75 % w/v | 90-95 % | 1. Chitosan (0.75 % w/v), starch (1.5% w/v), softener (.2 % w/v) 2. Chitosan (.75 % w/v), starch (1.5 % w/v), softener (.2 % w/v), clove oil (0.05 % w/v) 3. Chitosan (.75 % w/v), starch (1.5 % w/v), softener (.2 % w/v), turmeric (.5 % w/v) | Multifilament - polyethylene terephthalate (PET) - polyamide (Nylon 6) | Staphylococcus aureus (Gram-positive) | POSITIVE (ZI): All coated sutures are effective against S. aureus. |
| Hassan Mohammadi et al., 2020 | Hyaluronic acid Chitosan | Variable concentration | 75-85% | 1. Hyaluronic Acid (HA) (8% w/v) 2. Chitosan (4% w/v) 3. Chitosan (4% w/v) Hyaluronic Acid (HA) (8% w/v) | Suture with nylon monofilaments | Escherichia coli (Gram-negative) Staphylococcus aureus (Gram-positive) | POSITIVE NB=Number of bacteria (CFU/ml) Lower against S. aureus with a chitosan coating (4% w/v) - hyaluronic acid (8% w/v) Lower against E. coli with a chitosan coating (4% w/v) - hyaluronic acid (8% w/v) |
| Subramani Prabha et al., 2020 | Chitosan extracted from crab shells (EC) Commercial chitosan (CC) | Variable concentration for CC and EC | ------------ | 1. Chitosan (EC) 2. Chitosan (CC) 3. Vehicle control (VC) | Absorbable braided Vicryl suture | Staphylococcus epidermidis (Gram-positive) Candida albicans (Gram-negative) | POSITIVE (MIC) EC (200 µg/ml) showed a clear zone of inhibition for both test strains. CC (200 µg/ml) showed a minimal zone of inhibition for both test strains. |
Table 2 describes the process of adding chitosan alone or with an additional compound such as collagen, acetic acid, or hyaluronic acid to the suture surface. The process generally involves dissolving the chitosan powder in 1% acetic acid at 60 ºC and neutralizing it with 1% NaOH, curing, and drying.
Table 2: Coating evaluation and antimicrobial evaluation method
| Author/ Year | How chitosan was added to the sutures/Description | Method for measuring antibacterial activity | Description of the method for measuring antibacterial activity |
| Ying Yang et al., 2016 | Two groups of sutures were prepared; 1) GV (Gentamicin-coated Vicryl); 2) HV (Hydroxypropyltrimethylammonium chloride chitosan (HACC)-coated Vicryl suture). The coating process consisted of dissolving type I collagen in 5 mM acetic acid at a concentration of 0.5 mg/mL according to the manufacturer’s protocols. Then, gentamicin or HACC with a mass concentration of 0.2 wt% was dissolved in 50 mL of prepared collagen solutions and sonicated in an ultrasonic bath at 150 W (B3500S-MT, Branson, Shanghai, China, 50 Hz) for 5 minutes to obtain uniformly dissolved solutions. Then, Vicryl absorbable sutures were immersed overnight in the solutions to form the antibacterial agent-coated sutures as previously described. After collagen adsorption, the sutures were dried at room temperature for 24 hours. In each group, the same suture size was used for the in vitro and in vivo experiments, and all the tested sutures were cut into 2.5 cm in length for the in vitro assay (except for the 10-cm sutures used for the in vitro drug release). All prepared GV and HV sutures were sterilized with 25 kGy of 60 Co irradiation before conducting the biological experiments. | Zone of inhibition assay to compare antimicrobial effectiveness. Spread plate assay to evaluate bacterial addition and biofilm formation. Plate culture assay to detect biofilm formation. | ZI/bacterial suspension at a concentration of 1x108 CFUs/mL in MHB medium was inoculated onto the 10 cm tryptic soy agar (TSA) plate to obtain a uniform bacterial overlay (S. epidermidis (ATCC35984) and S. aureus (MRSA, ATCC43300)). Then, the prepared sutures were placed on the bacterial overlay and gently pressed in. The zones of inhibition of all the tested sutures were photographed and calculated after 24 hours of incubation at 37°C. |
| Faten Debbabi et al., 2017 | The chitosan was gradually dissolved by stirring in a 1% acetic acid solution and gradually heated to 60°C. Then, citric acid (CA) was slowly added to the chitosan dissolution. CA was used as a crosslinking agent between chitosan macromolecular chains. Finally, sodium hypophosphite was added as a catalyst of the crosslinking reaction. The second step of antibacterial suture manufacturing (braided suture made of 16 non-texturized PA 6-6 threads with 44 dtex count and 16 filaments per thread) was the PAD dry process to prevent the sutures from sticking together and to ensure a uniform coating. In the first step of the process, the braided suture was supported by braid roll and passed through the tension sensor. It was then placed in the coating bath containing the chitosan-CA solution. Then, the suture passed through a heat chamber of heat-setting machine. After that, the suture was neutralized in a 0.1N NaOH solution before being washed in a water bath. Finally, it was dried at 80°C and collected on a take-up spool. | Agar disk-diffusion method | First, the bacterial strains were cultured in a non-inhibitory agar gel to obtain isolated colonies. Then, after incubation at 37 ºC overnight, 4 to 5 well-isolated colonies were transferred to saline solution in Petri dishes. Sterilized sutures were then placed on the Petri dishes. The Petri dishes were then incubated at 37°C for 24 h. Finally, the mean distance of the inhibition growth zones (clear zone around the suture sample) was measured. The average inhibition distance of a 4 cm suture sample was determined against four bacterial strains: S. aureus, E. coli, Gram-positive S. epidermidis, and Gram-negative P. aeruginosa. In each case of bacterial strain, three samples were taken from each suture to determine the average value. |
| Masood et al. 2017. | Coating was carried out on a thread sizing machine (SS-565, CCI Technology Incorporation Taiwan) using hydrolyzed chitosan (15 g/l), previously dissolved in 1% acetic acid in demineralized water for 2 hours and then with HCl (35 ml/l). The chitosan solution was then heated under a reflux for 5 hours, cooled overnight, and filtered to remove any residual impurities. The temperature of the coating solution was maintained at 70°C. Drying conditions were kept at 100°C for 2 minutes and curing was performed at an elevated temperature of 130°C for 3 minutes using the laboratory oven. The cured sutures were rinsed with a 1% NaOH solution to remove any traces of hydrochloric acid and acetic acid and rinsed again with water and dried. | Test method AATCC 147-1998 | A 1 x 10-5 dilution of S. aureus (Strain ATCC 29213) that had been incubated overnight was spread on sterile agar plate. Test specimens of each of the coated and uncoated threads were placed gently transversely against the agar surface to ensure close contact. After 16 hours incubation at 37°C, the agar plates were examined for inhibition of microbial growth underneath the specimen and for the zone of inhibition along the edges of the specimen. |
| Hassan Mohammadi et al., 2020 | Nylon monofilaments (NMy) were coated with chitosan solution with hyaluronic acid (CH-AH) of different concentrations for 24 h at room temperature. They were first coated with chitosan and then treated in hyaluronic acid solutions (CH/HA-NMy) of different concentrations. Due to neutralization of the acetic acid content from the coated film, the nylon monofilaments were washed with 0.1 M NaOH solution and deionized water. The nylon monofilaments were subsequently padded and cured in the curing chamber at 100°C for 10 min. | Shake flask method | Three-centimeter samples were placed in 1 mL of PBS and incubated at 37 ºC for 5, 24, 48, and 72 h. E. coli as a gram-negative bacteria and S. aureus as a gram-positive bacteria were cultured overnight at 37°C. At each time point, the number of bacteria (CFU/mL) in the culture medium containing samples was determined. |
| Subramani Prabha et al., 2020 | Vicryl absorbable suture was UV sterilized and cut into small 1-cm pieces aseptically. The pieces were soaked in a solution of chitosan extract (EC) and commercial chitosan (CC) at 1 mg/mL, previously dissolved in 1% (v/v) acetic acid for approximately 12 h. | Agar disk diffusion method | The impregnated sutures were then air dried and placed on Petri-dishes swabbed with overnight culture of S. epidermidis and C. albicans and incubated at 37 °C for 24 h. After incubation, a clear zone of inhibition was observed around the suture sample. |
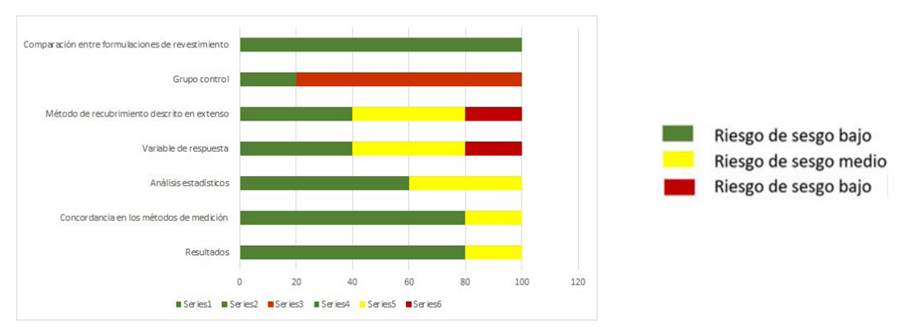
Table 3 and Figure 2 present the results of the risk of bias after comparing the included articles. In some of these studies, the authors did not always provide sufficient information or details to ascertain the quality of the methodology, and most authors did not include a control group. This influenced the values obtained and increased the bias. Table 3 shows the results: one article with moderate evidence and four with a high level of evidence.
Table 3: Quality analysis of the studies included for the summary of results
| Author | Comparison of coating formulations 0= None 1= One 2= Two or more | Control group 0= No control group 2= Control group | Coating method described in full 1= Not described 2= Partially described 3= Comprehensive description | Response variable 1= Qualitative subjective 2= Qualitative objective 3= Quantitative | Statistical analysis 0= Not clear 1= Partial 2= Complete | Concordance in measurement methods 0= None 1= Not clear 2= Present | Results 0= Incomplete 1= Partial 2= Complete | Total |
| Ying Yang et al., 2016 | 3 | 0 | 2 | 3 | 1 | 2 | 2 | 13 |
| Faten Debbabi et al., 2017 | 3 | 0 | 3 | 3 | 2 | 2 | 2 | 15 |
| R. Masood et al., 2017 | 3 | 0 | 2 | 1 | 1 | 2 | 1 | 10 |
| Hassan Mohammadi et al., 2020 | 3 | 0 | 3 | 2 | 2 | 1 | 2 | 13 |
| Subramani Prabha et al., 2020 | 3 | 2 | 1 | 2 | 2 | 2 | 2 | 14 |
| Total | 15 | 2 | 11 | 11 | 8 | 9 | 9 | 65 |
Level of Evidence: High= 13-17 points; Moderate= 9-12 points; Low= 5-8 points; Very low= 1-4 points
4. Discussion
Ming Kong et al. 21 reported that the antimicrobial effect of chitosan is influenced by four categories of factors: (1) microbial factors related to microorganism species and cell age; (2) intrinsic factors of chitosan like molecular weight, solubility, degree of deacetylation, positive charge density and concentration; (3) physical state of chitosan, i.e., water-soluble and solid state of chitosan; and (4) environmental factors, including pH, temperature, and reactive time. In this review, we only evaluated two of them: degree of deacetylation and concentration (Table 1). The degree of deacetylation indicates the total amount of acetamide groups converted to amine, which directly determines physical properties such as solubility, basicity, adsorption, and functionality of chitosan. A higher degree of deacetylation is associated with higher solubility and proton charge density. These two factors are essential for chitosan to adhere to the cell wall of microorganisms and to improve its antibacterial properties. In the studies included in this review, deacetylation degrees higher than 70% were used (Table 3).
Hassan Mohammadi et al.19 indicate that adding hyaluronic acid led to a decrease in surface roughness on the sutures, as the increase in the concentration of the chitosan solution (4% w/v) led to the formation of a uniform coating layer, which could be exfoliated. Adding a hyaluronic acid layer on the chitosan-coated thread leads to interaction between two polymers, preventing the coated layer’s exfoliation.18 Faten Debbabi et al. included citric acid to improve the friction and tensile properties at the knot of the suture. The results indicate that the knot tensile strength was higher than 17.4 N after coating, which is a limit on the minimum average required by the USP (United States Pharmacopeia Convention). However, a slight increase in the mean FCD (coefficient of friction) was observed as the concentration of chitosan and AC increased. These sutures are easier to handle and are better for knot tying.
Ying Yang et al.20 added hydroxypropyltrimethylammonium chloride to improve bio- and cytocompatibility properties. The results show that cell proliferation measured with the CCK-8 assay was similar in the four groups during the 120-hour culture period (p> 0.05). In addition, the cells exhibited good viability according to flow cytometry results.
The minimum inhibiting concentration (MIC) refers to the lowest concentration (in μg/mL) that inhibits the growth of a given bacterial strain. Subramani Prabha et al.17 concluded that the MIC range was 400 to 800 µg/mL for both EC (extracted chitosan) and CC (commercial chitosan) against the test strains. When comparing the MIC values of S. epidermidis and the mixed species, it was found that the MIC of CC was high for mixed species. Therefore, it was evident that microbes in mixed species have more robust membrane stability, which requires a higher concentration of antibacterial agents to lyse them. The other authors included did not determine MIC; antimicrobial activity was measured by ZI and CT. Antimicrobial activity decreases with increasing chitosan concentration. Hassan Mohammadi et al.’s19 coatings showed positive antimicrobial activity against Gram-positive and Gram-negative bacteria. It is worth mentioning that chitosan has antimicrobial properties mainly due to a positively charged amino group at a pH below 6.3 (carbon 2). This group interacts with the negative charges of the cell wall of the microorganisms, creating a rupture or lysis of these structures. This leads to the loss of protein compounds and other intracellular constituents.23 This is why chitosan shows greater activity against Gram-negative bacteria. Based on the results obtained by Hassan Mohammadi et al.,19 hyaluronic acid has no antibacterial properties in itself. It seems that its existence applies a synergistic reaction to chitosan and increases its activity against Gram-positive bacteria. Finally, all the included studies determined that the antimicrobial activity of chitosan increased when using coatings with low chitosan concentrations but an increased number of layers.
5. Conclusion
This review concluded that chitosan-coated sutures used in wound and incision closure would not be as susceptible to infection. This could help accelerate wound healing by preventing adherence and colonization of microbial communities. Adding other materials to this coating helps to improve the suture’s physical and biological properties. However, there is still insufficient evidence and records of in vivo studies and their positive outcomes.
Acknowledgments
The authors thank to Dr. René García Contreras for his advice throughout the project.
REFERENCES
1. Fakhri E, Eslami H, Maroufi P, Pakdel F, Taghizadeh S, Ganbarov K, Yousefi M, Tanomand A, Yousefi B, Mahmoudi S, Kafil HS. Chitosan biomaterials application in dentistry. Review. Int J Biol Macromol 2020;1(162):956-974. [ Links ]
2. Frank LA, Onzi GR, Morawski AS, Pohlmann AR, Guterres SS, Contri RV. Chitosan as a coating material for nanoparticles intended for biomedical applications. Reactive and Functional Polymers. 2020; (147):104459. [ Links ]
3. Cicciú M, Fiorillo L, Cervino G. Chitosan use in dentistry: systematic review of recent clinical studies. Mar Drugs 2019;17(7):417. [ Links ]
4. Chenxi Z, Didi H, Colin D, Huan S, Wei P, Xiaobing P, Zhengyong L, Jianxun S, Changchun Z. Preparation and application of chitosan biomaterials in dentistry. Review. Int J Biol Macromol 2021;15(167):1198-1210. [ Links ]
5. Poblete F, Dallaserra M, Yanine N, Araya I, Cortés R, Vergara C, Villanueva J. Incidencia de complicaciones posquirúrgicas en cirugía oral. Int J Interdiscip Dent 2020; 13(1):13-16. [ Links ]
6. Despaigne I, Rodríguez Z, Pascual M, Lozada GA, Mustelier HL. Consideraciones actuales sobre las infecciones posoperatorias. Review. MEDISAN. 2013;17(4): 686-707. [ Links ]
7. Lestari W, Yusry WN, Haris MS, Jaswir I, Idrus E. A glimpse on the function of chitosan as a dental hemostatic agent. Japan Dental Science Review 2020;56(1):147-154. [ Links ]
8. Viju S, Thilagavathi G. Effect of chitosan coating on the characteristics of silk-brained sutures. Journal of Industrial Textiles 2012;42(3):256-268. [ Links ]
9. Minozzi F, Bollero P, Unfer V, Dolci A, Galli M. The sutures in dentistry.Eur Rev Med Pharmacol Sci 2009;13(3):217-26. [ Links ]
10. Shlomo M, Avital K, Anda KS, Levartovskya Y, Mazora H. The effect of commonly used sutures on inflammation inducing pathogens - An in vitro study. J Craniomaxillofac Surg 2013;41(7):593-7. [ Links ]
11. Xiaojie C, Dandan H, Xiaoqi T, Lu W. Quantitative physical and handling characteristics of novel antibacterial braided silk suture materials. J Mech Behav Biomed Mater 2015;(50):160-70. [ Links ]
12. Ayala G. Efecto antimicrobiano del quitosano: una revisión de la literatura. Scientia Agroalimentaria. 2015;(2):32-38. [ Links ]
13. Clayton EM, Todd M, Dowd JB, Aiello AE. The impact of bisphenol A and triclosan on immune parameters in the U.S. population, NHANES 2003-2006. Environ Health Perspect. 2011;119(3):390-6. [ Links ]
14. Serrano C, García L, Fernández JP, Barbeck M, Ghanaati S, Unger R, Kirkpatrick J, Arzt E, Funk L, Turon P, del Campo A. Nanoestructured medical sutures with antibacterial properties. J Biomaterials. 2015;(52):291-300. [ Links ]
15. Hutton B, Ferrán C, López F., Moher D. La extensión de la declaración PRISMA para revisiones sistemáticas que incorporan metaanálisis en red: PRISMA-NMA. Medicina. 2016;(16):262-266. [ Links ]
16. Masood R, Hussain T, Umar M, Azeemullah, Areeb T, Riaz S. In situ development and application of natural coatings on non-absorbable sutures to reduce incision site infections. J Wound Care. 2017;26(3):115-120 [ Links ]
17. Prabha S, Sowndarya J, Ram PJVS, Rubini D, Hari BNV, Aruni W, Nithyanand P. Chitosan-Coated Surgical Sutures Prevent Adherence and Biofilms of Mixed Microbial Communities. Curr Microbiol 2021;78(2):502-512 [ Links ]
18. Debbabi F, Gargoubi S, Hadj Ayed MA, Abdessalem SB. Development and characterization of antibacterial braided polyamide suture coated with chitosan-citric acid biopolymer. J Biomater Appl 2017;32(3):384-398. [ Links ]
19. Mohammadi H, Alihosseini F, Hosseini SA. Improvement of the physical and biological properties of monofilament nylon as chitosan/hyaluronic acid suture. Int J Biol Macromol 2020; 164:3394-3402. [ Links ]
Conflict of interest declaration: The authors have no conflict of interest regarding the publication of this paper
Authorship contribution 1. Conception and design of study 2. Acquisition of data 3. Data analysis 4. Discussion of results 5. Drafting of the manuscript 6. Approval of the final version of the manuscript. PMKDG has contributed in 1, 2, 3, 4, 5, 6. OUMA has contributed in 2, 3, 4, 6. AAMC ha contribuido en 1, 2, 3, 4, 6.
Received: December 28, 2021; Accepted: July 19, 2022










 texto em
texto em