Servicios Personalizados
Revista
Articulo
Links relacionados
Compartir
Odontoestomatología
versión impresa ISSN 0797-0374versión On-line ISSN 1688-9339
Odontoestomatología vol.24 no.40 Montevideo dic. 2022 Epub 01-Dic-2022
https://doi.org/10.22592/ode2022n40e226
Research
Effects of xylitol on bacterial growth against Streptococcus sanguinis: In vitro study
1Universidad Nacional del Altiplano, Facultad Ciencias de la Salud. Escuela Profesional de Odontología. Puno, Perú.
2Universidad Autónoma de Barcelona, Maestría en Ingeniería Biológica y Ambiental. Barcelona, España.
Streptococcus sanguinis forms part of the oral biofilm, has a decisive role in the development of prevalent oral diseases and acts as an opportunistic pathogen at the systemic level.
Aims:
To evaluate in vitro the effects of xylitol on bacterial growth against Streptococcus sanguinis (ATCC 10556).
Methods:
The study sample was distributed into 6 groups: 4 experimental groups (1M; 0,75M; 0,50M and 0,25M xylitol), a negative control (distilled water) and a positive control (chlorhexidine). The statistical analysis was done using the statistical software Infostat and the tests used t-Student, ANOVA and Tukey to test the hypothesis.
Results:
different concentrations of xylitol (0,25M; 0,50M; 0,75M and 1M) caused an inhibition halo between 9,89 - 12,89 mm (24 hours) and 10,85 - 13,45 mm (48 hours).
Conclusions:
different concentrations of xylitol inhibit the bacterial growth of Streptococcus sanguinis, this inhibitory effect increases with higher concentration and exposure time.
Palabras clave: Xylitol; Streptococcus sanguinis; Bacterial Growth
Streptococcus sanguinis forma parte del biofilm bucal, tiene función decisoria en el desarrollo de las enfermedades bucales prevalentes y a nivel sistémico actúa como patógeno oportunista.
Objetivo:
Evaluar in vitro los efectos del xilitol en el crecimiento bacteriano frente a Streptococcus sanguinis (ATCC 10556).
Métodos:
la muestra del estudio fue distribuida en 6 grupos: 4 grupos experimentales (xilitol 1M; 0,75M; 0,50M y 0,25M), un control negativo (agua destilada) y un control positivo (clorhexidina); el análisis estadístico se hizo mediante el software estadístico Infostat y se empleó las pruebas t-Student, ANOVA y Tukey para contrastar la hipótesis.
Resultados:
diferentes concentraciones de xilitol (0,25M; 0,50M; 0,75M y 1M) causaron un halo de inhibición entre 9,89 - 12,89 mm (24 horas) y 10,85 - 13,45 mm (48 horas).
Conclusiones:
diferentes concentraciones de xilitol inhiben el crecimiento bacteriano del Streptococcus sanguinis, este efecto inhibitorio aumenta a mayor concentración y tiempo de exposición.
Palabras clave: Xilitol; Streptococcus sanguinis; Crecimiento Bacteriano
Streptococcus sanguinis faz parte do biofilme oral, tem papel decisivo no desenvolvimento de doenças bucais prevalentes e atua como patógeno oportunista em nível sistêmico.
Objetivo:
Avaliar in vitro os efeitos do xilitol no crescimento bacteriano contra Streptococcus sanguinis (ATCC 10556).
Métodos:
A amostra do estudo foi distribuída em 6 grupos: 4 grupos experimentais (1M; 0,75M; 0,50M e 0,25M xilitol), um controle negativo (água destilada) e um controle positivo (clorexidina); a análise estatística foi feita com o software estatístico Infostat e os testes t-Student, ANOVA e Tukey para testar a hipótese.
Resultados:
diferentes concentrações de xilitol (0,25M; 0,50M; 075M e 1M) causou um halo de inibição entre 9,89 - 12,89 mm (24 horas) e 10,85 - 13,45 mm (48 horas).
Conclusões:
diferentes concentrações de xilitol inibem o crescimento bacteriano de Streptococcus sanguinis, este efeito inibitório aumenta com maior concentração e tempo de exposição.
Palavras-chave: Xilitol; Streptococcus sanguinis; Crescimento bacteriano
Introduction
The oral cavity is a natural route of entry for bacteria both into the respiratory and digestive tracts and the blood stream.1 The oral microbial habitat of human beings consists of soft and hard surfaces that provide various opportunities for microbial colonization. These surfaces show variability depending on anatomical features, nutrient availability, temperature, oxygen concentration, and exposure to immunologic factors.2 Some studies indicate that a loss of balance in the symbiotic relationship between the oral microbiome and the host can be linked to certain diseases such as alveolar osteitis, tonsillitis, brain abscesses, endocarditis, liver abscesses, pneumonia, diabetes, and premature birth.1,3,4
The bacteria that make up the oral biofilm have a decisive role in the development of the most prevalent oral diseases such as dental caries and periodontal disease.1,3,5,6 Streptococci occupy a wide range of oral habitats that include sites without plaque where they are more abundant and they can be effective colonizers.2,7Streptococcus mitis, Streptococcus sanguinis, and Streptococcus gordonii are early colonizing bacteria which result in the formation of biofilm on the tooth surface, which is followed by the late colonization by pathogenic bacteria such as Streptococcus mutans, Veillonella spp., and Fusobacteria spp.7
Streptococcus sanguinis is a Gram-positive, facultative anaerobic commensal bacterium which is abundant in oral biofilm and is particularly associated with healthy plaque biofilm.3,5,8 It is a primary colonizer of oral biofilm that favors the attachment of successive organisms.4,6) Nine months is the average age of colonization with S. sanguinis in children.3,9 Biofilm formation begins when it attaches through fimbriae to multiple salivary components, including salivary α-amylase.3,8 Attaching to salivary components like salivary α-amylase can help S. sanguinis to bind to the hydroxyapatite in tooth surfaces and begin the formation of biofilms in the oral cavity.3 It can use a broad range of carbohydrate sources for survival.3 At the systemic level, when Streptococcus sanguinis enters the bloodstream, it can act as an opportunistic pathogen. Additionally, if it can colonize a damaged heart valve, it might lead to infective endocarditis.10
Xylitol is a naturally occurring sugar alcohol mainly derived from birch and other hardwood trees.11 It is found in some fruits and vegetables and has been approved in many countries as a sugar substitute. It is currently added as a sweetener to several commercial products such as chewing gum, candies, cosmetics, and oral hygiene products.12 It is also low in calories, not metabolized by most oral bacteria, and has anticariogenic properties.11,13 Although little is known about the mechanism of action of xylitol on pathogenic bacteria, evidence supports its preventive effect against several diseases, especially dental caries.11,12 The tolerable daily dose of xylitol is up to 200 g in adults and 45 g in children, with 4 to 20 g being the daily dose used to prevent dental caries.14 Its short-term use is associated with a reduction of Streptococcus mutans in saliva, in biofilm, and in motherchild transmission.11
This study evaluated the effects of xylitol on bacterial growth in vitro against Streptococcus sanguinis (ATCC 10556).
Methods
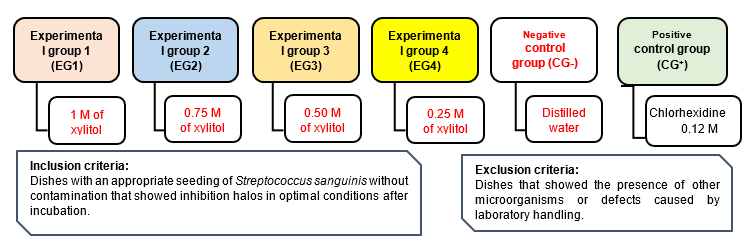
This was a prospective, longitudinal study with a quasi-experimental design. The sampling frame consisted of 105 replicate inoculations of Streptococcus sanguinis strains contained in 15 petri dishes, with 21 replicates in each group (Chart 1).
The Streptococcus sanguinis microorganism (ATCC 10556) was obtained
from the laboratory Gen Lab del Perú S.A.C. This study was conducted ethically and following the specifications contained in the Certificate of Analysis: specifications and yield of freeze-dried microorganisms issued by the supplying laboratory. The relevant biosafety measures were taken at all times to avoid bacterial contamination.
Samples were prepared by dissolving 1 M (152.15 g) of xylitol in 100 ml of distilled water. Afterwards, different volumes were obtained and placed in sterile test tubes labeled for each experimental group. For EG1, we took 10 ml of the solution (1 M/100 ml); for EG2 we took 7.50 ml of the solution (1 M/100 ml) and added 2.50 ml of distilled water; for EG3 we took 5 ml of the solution ( 1M/100 ml) and added 5 ml of distilled water; and for EG4 we took 2.50 ml of the solution (1 M/100 ml) and added 7.50 ml of distilled water.
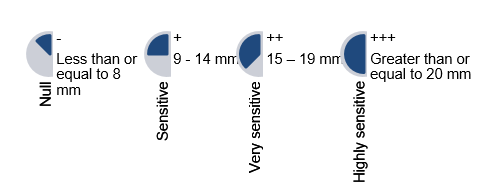
We prepared the Mitis salivarius (Difco Agar Mitis Salivarius) agar culture médium to activate and seed the Streptococcus sanguinis. The streaking method was used for the seeding, and the agar diffusion method by Kirby-Bauer was used for the microbial susceptibility test.15 Once the filter paper discs were positioned, each experimental group received 10 µl of the 1 M; 0.75 M; 0.50 M, and 0.25 M xylitol solutions, respectively. The negative control group was administered 10 µl distilled water, and the positive control was administered 10 µl chlorhexidine 0.12 M. Once the petri dishes were sealed and labeled, they were placed in the incubator at a temperature of 37°C for 24 and 48 hours before testing. The inhibitory effect was determined using the Duraffourd scale13 (Chart 2).
Excel and Infostat were used for statistical processing and data analysis. The difference between the mean of the averages and the variability of the inhibition halo between the different groups, as well as the significant difference between the groups according to exposure time, were calculated using the Student’s t-test for one sample, the statistical analysis of variance (ANOVA) and Tukey comparison test.
Results
Different xylitol concentrations (0.25 M; 0.50 M; 0.75 M, and 1 M) caused inhibition halos in the growth of Streptococcus sanguinis of between 9.89-12.89 mm (24 hours) and 10.85-13.45 mm (48 hours), the size of this inhibition halo increased with longer exposure time. The negative control maintained the measurement of the sensitivity disc at 6 mm, and the positive control produced an inhibition halo of 16.1919.80 mm after 24 and 48 hours (Table 1).
Table 1: In vitro comparison of the effects of xylitol on bacterial growth against Streptococcus sanguinis at 24 and 48 hours
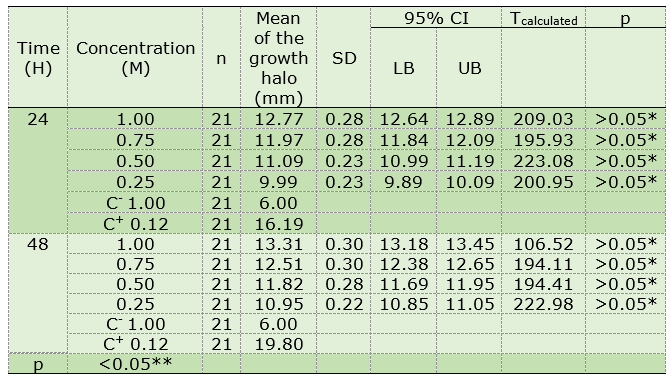
SD: standard deviation; CI: confidence interval; LB: lower bound; UB: upper bound; C-: Negative control, C+: Positive control, *Significance of p (one-sample Student’s t), **Significance of p (ANOVA).
The one-sample Student’s t-test showed that the data related to the inhibition halos in the growth of Streptococcus sanguinis was homogeneous in the groups with high xylitol concentrations (0.25 M; 0.50 M; 0.75 M, and 1 M) at 24 and 48 hours (p > 0.05*). The statistical analysis of variance (ANOVA) between the measurements of all of the studied groups showed a significant statistical difference in the inhibitory effects on the bacterial growth of Streptococcus sanguinis (coefficient of variation 2.18 and p < 0.05**). Therefore, the groups were compared using the Tukey test, the result of which was favorable for the positive control group followed by the xylitol solutions of 1 M (48 hours); 1 M (24 hours) and 0.75 M (48 hours); 0.75 M (24 hours) and 0.50 M (48 hours); 0.50 M (24 hours) and 0.25M (48 hours); and 0.25 M (24 hours) (alpha = 0.05; MSD = 0.28 and df = 200). This means that different xylitol concentrations inhibit the growth of Streptococcus sanguinis at 24 and 48 hours. In addition, the higher the xylitol concentration, the greater the inhibition effect on growth.
Discussion
In the oral cavity, microorganisms have a symbiotic ability and a relationship with the host that is based on mutual favors, such as not causing oral harm and allowing commensal populations to restrict the adhesion of pathogenic species to surfaces in the oral cavity.7 Species of the genus Streptococcus are primarily found in the surfaces of the oral mucosa,2,3 in human saliva,1,3 on tooth surfaces, and at the supragingival and subgingival levels.3,9 Some studies describe Streptococcus sanguinis as a species significantly associated with dental health.3,5,9 Together with Streptococcus mutans, they are an essential part of dental biofilm, and they adversely affect each other during biofilm formation. Díaz et al.,5 Hu et al.,16 and Wen et al.17 demonstrated the influence of Streptococcus sanguinis on the expression of the virulence genes Streptococcus mutans.
There is scientific evidence of the effect of xylitol on Streptococcus mutans.18,19 Regular exposure to xylitol reduces the formation of dental biofilm and the levels of Streptococcus mutans.20 This dental biofilm is less adhesive due to decreased Streptococcus mutans counts and the levels of insoluble polysaccharides.21) Cobos et al.12 identify the remineralizing effects of xylitol on enamel.
This study shows an inhibitory sensitivity in the growth of Streptococcus sanguinis between different dissolutions of xylitol (0.25 M; 0.50 M; 0.75 M and 1 M) p** < 0.05. Similar results were obtained by Ghezelbash et al.,22 who used xylitol solutions (2% and 4% w/v) in distilled water and demonstrated, with statistical significance, a reduction in bacterial growth of 57% and 65%, respectively. They also showed that it has an inhibitory effect on biofilm production and adhesion of Streptococcus sanguinis (p < 0.01). Sahni et al.23 also demonstrated an inhibition of the growth of three strains of oral Streptococcus (S. mutans, S. salivarius and S. sanguinis) with statistical significance. All three strains were inhibited significantly at xylitol concentrations of 12.5% and higher; however, only S. mutans was inhibited significantly at a 1.56% xylitol concentration.
This, however, differs from what was shown by Bahador et al.,24 who report that xylitol consumption (70% w/w) in chewing gum reduces S. mutansandS. sobrinusin saliva but showed no statistical significance in the counts of S. sanguinisandS. mitis, a difference which is probably due to the design of the study (community intervention). Marttinen et al.25 also showed that there was no statistical difference in the growth of S. sanguinis affected by xylitol (5%).
Other studies which used various antimicrobial agents to measure the inhibitory effect in the growth of Streptococcus sanguinis are consistent with our results. Nasution et al.26 indicate that the star fruit leaf extract has statistically significant antimicrobial efficacy against Streptococcus sanguinis (p < 0.05), and Lyu et al.27 report that ursolic acid has statistically significant antimicrobial activity against common oral Streptococcus and antibiofilm activity against oral pathogenic bacteria (p < 0.05). In addition, Berniyanti and Mahmiyah28 indicated that Saponin Aloe Vera Linn could inhibit the growth of Streptococcus sanguinis, and Oda et al.29) concluded that sodium fluoride (2%) reduces the adhesion of streptococci to titanium and zirconia implant abutment surfaces (p < 0.01). Cheng et al.30 also found statistical differences where stannous fluoride-containing toothpaste (0.45%) favored the overgrowth of S. sanguinis in the biofilm (p < 0.05).
In conclusion, different concentrations of xylitol have an inhibitory effect on the growth of Streptococcus sanguinis both at 24 and 48 hours, with a more significant impact at 48 hours and at higher concentrations.
REFERENCES
1. Hasan NA, Young BA, Minard-Smith AT, Saeed K, Li H, Heizer EM, McMillan NJ, Isom R, Abdullah AS, Bornman DM, Faith SA, Choi SY, Dickens ML, Cebula TA, Colwell RR. Microbial community profiling of human saliva using shotgun metagenomic sequencing. PLoS OnE 2014; 9(5):97699. [ Links ]
2. Margarita S, Quintana C, Sjostrom IPD, Arias IID, Marlene IG, Baldeón M. Microbiota de los ecosistemas de la cavidad bucal Microbiota of oral cavity ecosystems. Rev Cubana Estomatol 2017;54(1):84-99. [ Links ]
3. Zhu B, Macleod LC, Kitten T, Xu P. Streptococcus sanguinis biofilm formation & interaction with oral pathogens. Future Microbiol (Internet) 2018;13(8):915-32. Disponible en: https://www.futuremedicine.com/doi/10.2217/fmb-2018-0043 [ Links ]
4. Hashizume-Takizawa T, Yamaguchi Y, Kobayashi R, Shinozaki-Kuwahara N, Saito M, Kurita-Ochiai T. Oral challenge with Streptococcus sanguinis induces aortic inflammation and accelerates atherosclerosis in spontaneously hyperlipidemic mice. Biochem. Biophys. Res. Commun. 2019;520(3):507-13. [ Links ]
5. Díaz-Garrido N, Lozano CP, Kreth J, Giacaman RA. Competition and Caries on Enamel of a Dual-Species Biofilm Model with Streptococcus mutans and Streptococcus sanguinis. Appl. Environ. Microbiol. (Internet) 2020 (cited 2021 Jul 3);86(21). Disponible en: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7580551/ [ Links ]
6. Oda Y, Miura T, Mori G, Sasaki H, Ito T, Yoshinari M, Yajima Y. Adhesion of streptococci to titanium and zirconia. PLoS One (Internet) 2020 (cited 2021 Jul 27);15(6):e0234524. Disponible en: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7314031/ [ Links ]
7. Mark-Welch JL, Rossetti BJ, Rieken CW, Dewhirst FE, Borisy GG. Biogeography of a human oral microbiome at the micron scale. PNAS (Internet). 2015 (cited 2022 Sep 18);113(6):e791-800. Disponible en: https://www.pnas.org/doi/pdf/10.1073/pnas.1522149113 [ Links ]
8. Lozano CP, Díaz-Garrido N, Kreth J, Giacaman RA. Streptococcus mutans and Streptococcus sanguinis Expression of Competition-Related Genes, under Sucrose. Caries Res. 2019; 53(2):194-203. [ Links ]
9. Giacaman RA, Torres S, Gómez Y, Muñoz-Sandoval C, Kreth J. Correlation of Streptococcus mutans and Streptococcus sanguinis colonization and ex vivo hydrogen peroxide production in carious lesion-free and high caries adults. Arch. Oral Biol. 2015;60(1):154-9. [ Links ]
10. Puccio T, Kunka KS, Zhu B, Xu P, Kitten T. Manganese Depletion Leads to Multisystem Changes in the Transcriptome of the Opportunistic Pathogen Streptococcus sanguinis. Front. Microbiol. 2020;11. [ Links ]
11. Misra S, Raghuwanshi S, Gupta P, Saxena RK. Examine growth inhibition pattern and lactic acid production in Streptococcus mutans using different concentrations of xylitol produced from Candida tropicalis by fermentation. Anaerobe 2012;18(3):273-9. [ Links ]
12. Cobos Ortega C, Valenzuela Espinoza E, Ángel Araiza M. Influencia de un enjuague a base de fluoruro y xilitol en la remineralización in vitro del esmalte en dientes temporales. Rev. Odont. Mex. 2013;17(4):204-9. [ Links ]
13. Checalla-Collatupa JL, Sánchez-Tito MA. Caracterización Química y Actividad Antibacteriana in vitro de un Extracto Etanólico de Propóleo Peruano Frente a Streptococcus mutans. Int. J. Odontostomat. 2021;15(1):145-51. [ Links ]
14. Makinen KK, Bennett CA, Hujoel PP, Isokangas PJ, Isotupa KP, H.R. Pape J, Makinen P. Xylitol Chewing Gums and Caries Rates: A 40-month Cohort Study: J. Dent. Res. 1995;74(12):1904-13. [ Links ]
15. Gajdács M, Spengler G, Urbán E. Identification and Antimicrobial Susceptibility Testing of Anaerobic Bacteria: Rubik's Cube of Clinical Microbiology? Antibiotics 2017;6(4). [ Links ]
16. Hu D, Gong J, He B, Chen Z, Li M. Surface properties and Streptococcus mutans - Streptococcus sanguinis adhesion of fluorotic enamel. Arch. Oral Biol. 2021;121:104970. [ Links ]
17. Wen ZT, Yates D, Ahn SJ, Burne RA. Biofilm formation and virulence expression by Streptococcus mutans are altered when grown in dual-species model. BMC Microbiol. 2010;10(1):111. [ Links ]
18. de la Cruz SB, Albites U. Efectividad de las pastas dentales en la reducción del recuento de Streptococcos mutans en niños de 5 años de edad. Odontol Pediatr (Internet) 2021 (cited 2021 Jul 3);19(2):33-9. Disponible en: http://www.op.spo.com.pe/index.php/odontologiapediatrica/article/view/133 [ Links ]
19. Escalante-Medina RP, Asmat-Abanto AS, Ruiz-Barrueto MA. Efecto antibacteriano de una pasta dental con xilitol sobre Streptococcus mutans en saliva de gestantes. Rev Cubana Estomatol (Internet) 2019;56(4):1-11. Disponible en: http://www.revestomatologia.sld.cu/index.php/est/article/view/1825 [ Links ]
20. Loimaranta V, Mazurel D, Deng D, Söderling E. Xylitol and erythritol inhibit real-time biofilm formation of Streptococcus mutans. BMC Microbiol. 2020 ;20(1). [ Links ]
21. Saheer PA, Parmar P, Majid SA, Bashyam M, Kousalya PS, Marriette TM. Effect of sugar-free chewing gum on plaque and gingivitis among 14-15-year-old school children: A randomized controlled trial. Indian J Dent Res 2019;30(1):61. [ Links ]
22. Ghezelbash GR, Nahvi I, Rabbani M. Comparative inhibitory effect of xylitol and erythritol on the growth and biofilm formation of oral Streptococci. Afr. J. Microbiol. Res. 2012;6(20):4404-8. [ Links ]
23. Sahni PS, Gillespie JM, Botto RW, Otsuka AS. Pruebas in vitro de xilitol como agente anticariogénico. Gen Dent 2002;50(4):340-3. [ Links ]
24. Bahador A, Lesan S, Kashi N. Effect of xylitol on cariogenic and beneficial oral streptococci: a randomized, double-blind crossover trial. Iran J Microbiol ,Internet, 2012 (cited 2021 Jul 24);4(2):75. Disponible en: https://pubmed.ncbi.nlm.nih.gov/22973473/ [ Links ]
25. Marttinen AM, Ruas-Madiedo P, Hidalgo-Cantabrana C, Saari MA, Ihalin RA, Söderling EM. Effects of Xylitol on Xylitol-Sensitive Versus Xylitol-Resistant Streptococcus mutans Strains in a Three-Species in Vitro Biofilm. Curr Microbiol 2012;65(3):237-43. [ Links ]
26. Nasution M, Simatupang Y, Dennis D. Effectiveness of star fruit leaf extract on the growth of streptococcus sanguinis: An in vitro study. World J. Dent. 2020;11(3):196-200. [ Links ]
27. Lyu X, Wang L, Shui Y, Jiang Q, Chen L, Yang W, He X, Zeng J, Li Y. Ursolic acid inhibits multi-species biofilms developed by Streptococcus mutans, Streptococcus sanguinis, and Streptococcus gordonii. Arch. Oral Biol. 2021;125:105107. [ Links ]
28. Berniyanti T, Mahmiyah E. Microbiological studies on the production of antimicrobial agent by Saponin aloe vera linn against Streptococcus sanguinis. Res. J. Microbiol. 2015;10(10):486-93. [ Links ]
29. Oda Y, Miura T, Hirano T, Furuya Y, Ito T, Yoshinari M, Yajima Y. Effects of 2% sodium fluoride solution on the prevention of streptococcal adhesion to titanium and zirconia surfaces. Sci. Rep. 2021;11:4498. [ Links ]
30. Cheng X, Liu J, Li J, Zhou X, Wang L, Liu J, Xu X. Comparative effect of a stannous fluoride toothpaste and a sodium fluoride toothpaste on a multispecies biofilm. Arch. Oral Biol. 2017;74:5-11. [ Links ]
Authorship contribution: 1. Conception and design of study 2. Acquisition of data 3. Data analysis 4. Discussion of results 5. Drafting of the manuscript 6. Approval of the final version of the manuscript RA has contributed in a,b,c,f. SA has contributed in a,b,c,f. TP has contributed in a,b,d,f. VM has contributed in d,e,f. PC has contributed in a,e,f. FA has contributed in b,c,f
Received: July 20, 2022; Accepted: November 22, 2022











 texto en
texto en