Serviços Personalizados
Journal
Artigo
Links relacionados
Compartilhar
Odontoestomatología
versão impressa ISSN 0797-0374versão On-line ISSN 1688-9339
Odontoestomatología vol.24 no.40 Montevideo dez. 2022 Epub 01-Dez-2022
https://doi.org/10.22592/ode2022n40e222
Research
Effect of the curing light intensity on the biocompatibility and flexural strength of a composite resin
1Cátedra de Materiales Dentales, Facultad de Odontología, Universidad de la República, Uruguay. ggrazioli@odon.edu.uy
2Cátedra de Patología y Semiología buco-maxilo-facial y Clínica Estomatológica, Facultad de Odontología, Universidad de la República, Uruguay
Objective:
To determine the effect of the intensity of two light curing units on the biocompatibility, flexural strength and elastic modulus of a composite resin.
Methodology:
Two groups of Filtek Z250XT (3M ESPE) composite resin were created, each one photopolymerized using different intensities (<400 mW/cm2 for 40s and> 800 mW/cm2 for 20s). Cell viability was analyzed by MTT assay at 24 and 48 hours following the ISO 10993-5 standard. The flexural strength and elastic modulus were analyzed following the ISO 4049 standard.
Results:
In the group photopolymerized with an intensity <400 mW/cm2, cytotoxicity was statistically higher both at 24 and 48 hours and flexural strength and elastic modulus were statistically lower.
Conclusion:
A polymerization intensity <400 mW/cm2 increases the levels of cytotoxicity and decreases the mechanical properties of composite resins. The importance of the periodic control of the light curing units is emphasized.
Keywords: materials testing; composite resins; cell culture
Objetivo:
Determinar el efecto de la intensidad de dos unidades de fotopolimerización sobre la biocompatibilidad, resistencia flexural y módulo elástico de una resina compuesta.
Metodología:
Se crearon dos grupos de resina compuesta Filtek Z250XT cada uno fotopolimerizado con intensidades diferentes (<400 mW/cm2 por 40s y >800 mW/cm2 por 20s). La viabilidad celular fue analizada mediante ensayo de MTT a las 24 y 48 horas siguiendo la normativa ISO 10993-5. La resistencia flexural y módulo elástico fueron analizadas siguiendo la normativa ISO 4049.
Resultados:
En el grupo fotopolimerizado con una intensidad <400 mW/cm2, la citotoxicidad fue estadísticamente mayor tanto a las 24 como a las 48 horas y la resistencia flexural y módulo elástico fueron estadísticamente menores
Conclusión:
Una intensidad de polimerización <400 mW/cm2, aumenta los niveles de citotoxicidad y disminuye las propiedades mecánicas de las resinas compuestas. Se destaca la importancia del control periódico de las unidades de fotopolimerización.
Palabras claves: Ensayo de Materiales; Resinas Compuestas; Cultivo de Células
Objetivo:
Determinar o efeito da intensidade de duas unidades de fotopolimerização na biocompatibilidade, resistência à flexão e módulo de elasticidade de uma resina composta.
Metodologia:
Foram fabricados dois grupos de resina composta Filtek Z250XT, cada um deles foi fotopolimerizado com intensidades diferentes (<400 mW/cm2 por 40s e > 800 mW/cm2 por 20s). A viabilidade celular foi analisada por ensaio de MTT em 24 e 48 horas seguindo a norma ISO 10993-5. A resistência à flexão e o módulo de elasticidade foram analisados seguindo a norma ISO 4049.
Resultados:
No grupo fotopolimerizado com intensidade <400mW/cm2, a citotoxicidade foi estatisticamente maior nas 24 e 48 horas e a resistência à flexão e o módulo de elasticidade foram estatisticamente menores.
Conclusão:
Uma intensidade de polimerização <400 mW/cm2 aumenta os níveis de citotoxicidade e diminui as propriedades mecânicas das resinas compostas. Destaca-se a importância do controle periódico das unidades de fotopolimerização.
Palavras-chave: Teste de Materiais; Resinas Compostas; Cultura de Células
Introduction
Composite resins (CR) are polymeric materials widely used in dental practice. They are indicated for direct and indirect filling, cementing restorations, and orthodontic brackets, among other applications. Their clinical performance is highly dependent on essential characteristics, such as good mechanical properties, easy handling, and non-toxic and non-irritating to dental tissues.1
Biocompatibility is the materials’ ability to coexist harmoniously with the surrounding biological environment.2 This implies that the restorative material must not harm the pulp or soft tissues, must not contain toxic substances that can be diffused, released and/or absorbed by the surrounding environment, nor cause allergic reactions or have carcinogenic potential.2,3 Multiple assays that measure the viability and proliferation status of cells exposed to test materials in vitro can be used to assess the relative toxicity of materials.2 According to the current international standard (ISO 10993-5),4 this assessment can be performed using MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromid). This is the most common test for assessing the cytotoxicity of dental materials as it is quick and inexpensive. It is based on cells reducing MTT to formazan crystals. Therefore, its concentration measured via spectrophotometry allows us to quantify the viable cells indirectly.2
Furthermore, (tensile, compressive, or flexural) mechanical strength values are used as parameters to evaluate the performance of these polymeric materials.5 Flexural strength (FS) is the mechanical property recommended by the International Organisation for Standardisation to study materials based on CR (ISO 4049:2009).6 This value is obtained with the three-point bending test, where the material bends until it fractures. In turn, this type of test can analyse the elastic modulus (EM) of the material, i.e., how stiff or flexible the material is. FS has been shown to be more selective and sensitive than compressive strength when there are subtle changes in a polymer substructure.7
RC hardens through polymerization: monomers are converted to polymers. This process occurs through photo-activation, which requires light-curing units (LCUs) at a specific wavelength and intensity. Currently, halogen and LED units are the most widely used, with a wavelength between 360 and 520 nm. They are effective for material polymerization using camphorquinone as a photoinitiator.8
It is expected that all the carbon double bonds (C = C) of the CR matrix monomer will convert to single bonds (C - C), which form the polymeric network.1 However, the conversion degree (CD) of monomers to polymers ranges from 55 to 75%,3 depending on the chemical structure of the monomers, filler type, material translucency, the thickness of the material to be polymerized, the time, intensity, density of the light and the distance between the LCUs and the material.9 The relationship between the wavelength and the photoinitiator system has a significant effect on the CD of the monomers. The literature shows that the right CD requires radiation exposure (RE) between 16-20 J/cm2.8 The RE is established by the power of the light (mW/cm2) multiplied by the exposure time (in seconds). Therefore, to achieve the right CD, we must establish a light exposure time according to the intensity of our LCU.10 A total energy of 16 J is necessary to achieve the right excitation of the photoinitiator. This RE is achieved, for example, by having a minimum light intensity of 400 mw/cm2 for an exposure time of 40 seconds.2
However, LCU intensity might decrease with lamp and filter deterioration, improper maintenance, battery wear, resin residues on the tip of the LCU, improper use of the LCU by placing the light source far away from the material to be polymerized, etc.8 This results in a decrease in the CD of the organic matrix, negatively affecting the physicomechanical properties due to reduced cross-linking of molecules.3,9 Therefore, it would be expected that the increase in residual monomers would lead to decreased biocompatibility, increasing their cytotoxic capacity after these monomers are released.
In clinical practice, it is essential to monitor LCUs periodically by evaluating the intensity of the light emitted with radiometers11 because it is impossible to determine LCUs lacking optimum intensity clinically, as even if the CRs are light-cured below 16 J, they feel rigid to the touch.2
Therefore, this study aimed to determine the effect of LCU intensity on CR biocompatibility and flexural strength. It also seeks to raise practitioners’ awareness of the importance of monitoring their LCUs periodically.
We posit the following hypothesis: CRs polymerized with LCU with an intensity <400 mW/cm2 would be more cytotoxic and have lower flexural strength and elastic modulus values than those cured with higher intensities.
Methodology
This study was approved by the Research Ethics Committee of the School of Dentistry, Universidad de la República, File No. 091900000154-18.
Two groups were created for this study according to LCU intensity:
- Group 1: GNATUS OPTILIGHT 600 (Gnatus; Ribeirao Preto, Brazil) with an intensity <400 mW/cm2 (exactly 340 mW/cm2).
- Group 2: GNATUS OPTILIGHT MAX LED unit (Gnatus; Ribeirao Preto, Brazil) with an intensity >800 mW/cm2 (exactly 860 mW/cm2).
LCU intensity previously measured using a Bluephase meter II radiometer (Ivoclar Vivadent; Schaan, Liechtenstein, Germany). The LCUs were in operation and showed different degrees of wear and tear at the time of the study.
A commercial RC Filtek Z250XT (3M ESPE, ST PAUL, MN, USA) was used to conduct the tests. It was polymerized with the LCU according to the relevant group, following the manufacturer’s instructions: 40 seconds with the light-curing unit with an intensity <400 mW/cm2 and 20 seconds with the light-curing unit with an intensity >800 mW/cm2. The manufacturer’s composition and instructions can be found in Table 1. A single trained operator manipulated all the material.
Table 1: Features of the Filtek Z250XT composite resin
| Commercial Name | Manufacturer | Composition | Manufacturer’s instructions |
| Filtek Z250XT (A2 color) | 3M ESPE | Matrix: Bis-GMA, Bis-EMA, TEGDMA, PEGDMA and UDMA. Filler: Nanohybrid (silica, zirconia, zirconia/silica cluster). 82% by weight. Silane. | For 2-mm material thickness: - lamps with a maximum intensity of 800 mW/cm2 and polymerization for 40 seconds. - lamps with an intensity higher than 800 mW/cm2 and polymerization for 20 seconds. |
Cytotoxicity analysis
Sample preparation
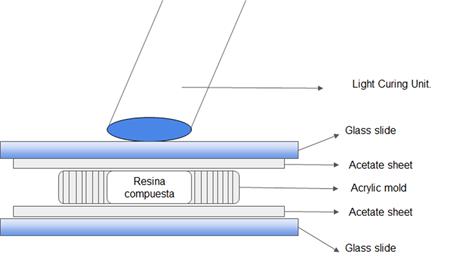
Five specimens were prepared for each group. Acrylic molds measuring 5 mm in diameter and 1 mm in depth were placed on an acetate sheet. The CR was inserted into the mold and an acetate sheet was pressed with a glass slide on top to ensure sample uniformity (Figure 1). Each specimen was polymerized according to the relevant group. Finally, they were removed from the molds and the edges were sanded with 400 grit sandpaper to remove any excess.
Cell culture
All culture procedures were carried out under strict biosafety standards in a FORMA 1300 A2 laminar flow chamber, Model 1386 (Thermo Scientific, Waltham, Massachusetts, Estados Unidos). NIH 3T3, an immortalized mouse fibroblast cell line, was used and previously cultured using clonogenic culture medium (DMEM + 10% Fetal Bovine Serum + 1% Penicillin/Streptomycin) (GIBCO, Denmark) in t25 culture flasks in an incubator (FORMA 311, Thermo Scientific), Waltham, Massachusetts, Estados Unidos) at 37ºC with 5% CO2 under relative humidity.
The material was transferred to a t75 culture flask when 80% confluence was reached. For this, the culture medium was removed, the cells were washed with phosphate buffer saline (PBS) (GIBCO, Denmark), then 1 ml trypsin/EDTA (TrypLE®, Thermofisher) was added for 5 minutes at 37°C. Finally, trypsin was neutralized with 2 ml of clonogenic medium, and the entire cell suspension was placed in a t75 culture flask, adding a clonogenic medium to continue cell expansion. The cell viability assay was started when 80% confluence was reached.
Cell viability assay
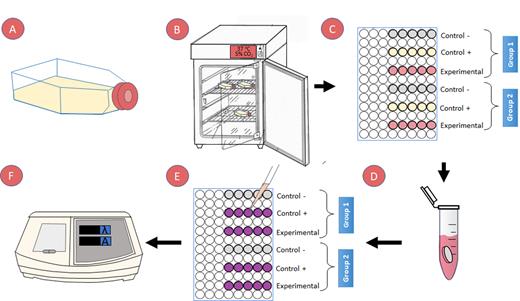
The cell viability assay was performed following a previously established protocol,12 complying with ISO 10993-54 (Figure 2). Each previously prepared specimen was placed in a 2 ml Eppendorf® type container, and 1 ml clonogenic medium was added and left to incubate for 24 hours. In this way, any toxic components contained in the sample were released into the culture medium by leaching (eluate).
The previously collected cells were streaked in 96-well plates at a density of 3x104 cells per well and incubated for 24 hours with 100 μl clonogenic medium. Thus we obtained six columns of five wells for each group: negative control without cells, positive control with cells and clonogenic medium, and experimental with the medium incubated in the specimens. After 24 hours, the DMEM was changed in the control wells, and 100 μl of the previously incubated medium (eluate) with the specimens was placed in the experimental wells.
Viability was measured at 24 and 48 hours. First, the culture medium was removed, and the wells were washed with PBS. Then, 100 μl of clonogenic medium supplemented with 5% MTT (GIBCO, Denmark) was added and incubated for 5 hours at 37°C and 5% CO2. Finally, the medium was removed, and 100 μl of dimethyl sulfoxide (DMSO, Sigma-Aldrich, Missouri, United States) was added for 5 minutes to reveal the precipitates under gentle agitation.
Cell viability was quantified with an ELISA ultraviolet visible spectrophotometer at 570 nm (Leytemed LTCM06 Elisa Microplate Reader, Guangzhou, China). This device was used to quantify the precipitates. These precipitates produce a purplish staining, which allows us to indirectly measure MTT metabolization, indicating the viability of the cultured cells. Once the values were obtained, they were analyzed using the following formula:
A) NIH 3T3 + clonogenic culture medium (DMEM + 10% FBS + 1% P/S), B) incubated to 80% confluence, C) Cell streaking in 96-well plates at a density of 3x104 with 100 μl clonogenic medium for 24 hours. D) Creation of eluate by incubating each specimen in 1 ml of clonogenic medium. E) Cell viability assay measured at 24 and 48 hrs: - add MTT solution for 4 hours. - aspirate MTT. - dilute the precipitate in DMSO and shake for 5 min to observe the precipitate. F) Quantification of cell viability with ELISA ultraviolet visible spectrophotometer at 570 nm.
Three-point bending test
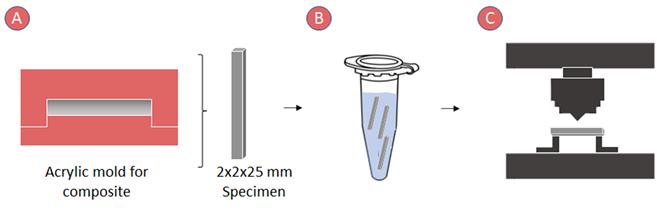
A three-point bending test was performed according to ISO 4049 to analyze the flexural strength and elastic modulus (Figure 3).6
Sample preparation
Ten rod-shaped specimens (25 x 2 x 2 x 2 mm) per group were made by placing the uncured resin in an acrylic mold covered with a strip of celluloid and pressing with a glass slide. The rods were polymerized following the overlapping method described in ISO 4049.6
After polymerization, they were removed from the mold, and the excess was removed using 400-grit sandpaper. Each specimen was measured using a digital caliper (Mitutoyo, Japan) with a 0.01 mm accuracy. Before mechanical testing, the samples were stored in distilled water at 37°C for 24 hours.
Mechanical test
The mechanical test was performed using an MTS SANS CMT2000 universal testing machine (Sans Testing Machine, Shen Zhen, China) with a load cell of 5 kN and a crosshead speed of 0.75 mm/s until fracture. The following equations were used to determine the flexural strength (σ) and the elastic modulus (E):
Where P is the load at fracture (N), l is the distance between the supports (20 mm), b is the width (mm), and h is the height of the specimen (mm), P1 is the maximum load in the linear portion (proportional limit) of the stress-strain curve, and d is the deformation of the specimen at P1 load.6
Statistical analysis
The cell viability results were analyzed with a two-way ANOVA test: a) LCU used and b) analysis time (24 or 48 hours). The results of flexural strength and elastic modulus were analyzed with the Student’s test. All the results were previously subjected to a normality test, and the significance level was α=0.05 in all the analyses. SigmaStat v3.5 (Systat Software, California, USA) was used for this statistical analysis.
Results
Cell viability
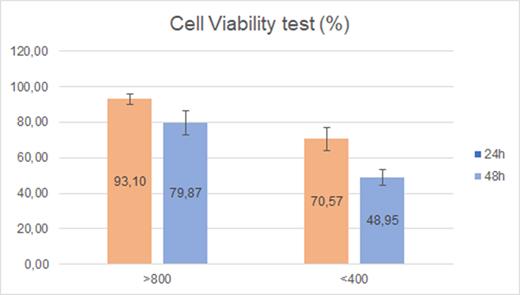
The results of cell viability analysis (Figure 4) show a statistically significant difference (p<0.001) between the groups studied, with higher degrees of cytotoxicity when using an intensity <400 mW/cm2. A two-way analysis revealed a statistical difference when assessing cytotoxicity at 24 and 48 hours (p<0.001).
Table 2: Table of two-way ANOVA results
| Variation source | Degrees of freedom | Sum of squares | Mean sum of squares | F | P-value |
| Intensity | 1 | 5000.374 | 5000.374 | 150.416 | <0.001 |
| Time | 1 | 2163.613 | 2163.613 | 65.083 | <0.001 |
| Intensity x Time | 1 | 132.134 | 132.134 | 3.975 | 0.057 |
| Residual | 25 | 831.091 | 33.244 | ||
| Total | 28 | 8477.913 | 302.783 |
Three-point bending test
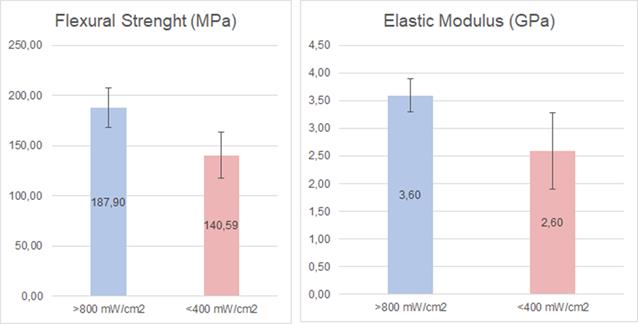
The analysis of the flexural strength (Figure 5) showed a statistically significant difference (p=0.02) between <400 and >800 mW/cm2.
The analysis of the elastic modulus (Figure 5) showed a statistically significant difference (p=0.037) between <400 and >800 mW/cm2. Higher flexural strength and elastic modulus values were obtained using a >800 mW/cm2 unit.
Discussion
This study evaluated the effect of the intensity of two LED LCUs on the mechanical and biological properties of a CR since their proper polymerization cannot be adequately verified. The results showed significant differences in cell viability, flexural strength, and elastic modulus, depending on the intensity used. The group that was light-cured with higher intensity showed statistically superior cell viability and mechanical properties than the group light-cured with a lower intensity unit. Therefore, our working hypothesis has been proven.
The biocompatibility of dental materials has been researched with different methods, such as tests to estimate the number of ribonucleic acids and damage to their chains,13 studies assessing the severity of proteolytic action,14 assessment of glutathione level in cells,15 and metabolic activity evaluation (e.g., MTT assays),9 among others. In this study, we selected an MTT assay because, despite the existence of other methods, it is a good indicator of cell viability, and it is the method indicated by current international standards to analyze biomaterials for dental use.4 This study showed a significant decrease in cell viability at 24 h (93.1% Group 2, 70.6% Group 1) and 48 h (79.8% Group 2, 48.5% Group 1) regarding cellular control (100%). These data are aligned with those found in the literature.9 It is relevant to consider that according to ISO 10993-5, the reduction of cell viability by over 30% is considered a cytotoxic effect.4
Cytotoxicity levels in resinous materials can be caused by gradual degradation over time as more toxic components are released into the environment. Therefore, this depends on the time and concentration of the main component of these materials: Bis-GMA.9 Several authors have analyzed the cytotoxicity of Bis-GMA in dental materials by exposing fibroblasts to different Bis-GMA concentrations.1,16 It has been demonstrated that there is a dose-dependent response over time, where the higher the Bis-GMA concentration, the lower the cell viability.3 Schubert et al. demonstrated that the cytotoxic effect of the material can also be significantly affected by its color shade. They observed higher cytotoxicity in C2-colored resins compared to A2 shades.15 This could be explained by the fact that the CD is affected by metallic pigments, such as Cu2+, Al3+, and Fe2+, used in the darker resin shades.17 Some studies have established that Bis-GMA is among the most toxic components due to its liposolubility, followed by UMDA, TEGDMA, and HEMA.18,19 In addition, synergistic effects were observed when combinations of TEGDMA with UDMA or particularly with Bis-GMA were tested.8 Gonçalves et al. compared the cytotoxic effect of conventional and flowable resins, showing that flowable resins were significantly more toxic than regular consistency resins. This could be because flowable resins contain more monomer and less filler.20 In vitro studies have shown that the polymerization reaction is never complete but ranges between 50 and 70% of the matrix.9 As explained above, this could be affected by an improper polymerization protocol, which decreases the CD.10,16
Regarding mechanical behavior, several studies have shown that insufficient intensity and energy significantly decrease the CD of CRs.19,21 Furthermore, a significant correlation exists between CD and mechanical properties such as hardness, elastic modulus, and flexural strength of CR.22 Therefore, we expected the results of this study to show that the group polymerized with an LCU >800 mW/cm2 had a superior mechanical behavior, since its CD would be higher compared to the group polymerized with an LCU <400 mW/cm2. Although filler size and polymer matrix composition can influence the CD,2,11 in this study, only one type of CR (Z250XT) was used. Therefore, the control for this variable shows that the difference in mechanical properties was due to the variation in LCU intensity; hence the CD decreases due to lower RE.10,21
On the other hand, some studies have shown that the elastic modulus decreases if linear molecules within the polymer structure are increased.23 Therefore, we can infer that the samples polymerized with an LCU <400 mW/cm2 presented a more linear polymeric structure than those polymerized with an LCU >800 mW/cm2, which presented a more branched structure. In addition, there is a relationship between CD, spatial structure, and aqueous sorption of CRs, which favors the diffusion of unreacted monomers into oral tissues increasing their toxicity.2 This could also explain the differences between the cytotoxicity of the two groups.
Regarding the adverse effects of incorrect polymerization, studies have shown a strict connection between the properties of the restorative material and its longevity.24 This would affect not only the mechanical behavior of the material but also its color stability and aesthetics.25 In addition, this would increase the CR solubility,26 causing higher surface roughness and biofilm formation,27 increasing the risk of caries. Therefore, it is not recommended to use LCU with an intensity <400 mW/cm2 to reduce the probability of CR damage.
The study’s limitations are that it is an in vitro assay, so clinical behavior may vary. Further clinical studies are needed to support these results. In addition, it should be noted that the study used only one CR, so the results are likely to vary in other CR types.
The data obtained in this study suggest that using an LCU PU with an intensity <400 mW/cm2 decreases the physico-mechanical and biological properties of CRs, thus causes a lower CD. This is clinically relevant because there is a relationship between the material’s properties and its clinical performance.22,24 It is essential to know the intensity of our LCU to establish the irradiation time and thus achieve an adequate CD.8,23. It has been reported that the light intensity of the LCU can be affected over time by the deterioration of the unit or dirty or chipped fiber tips,11,21,23 so it is essential to monitor LCUs periodically with a digital radiometer. Furthermore, it is important to know the manufacturer’s indications, the composition of the CRs, and the LCU’s wavelengths, so that its emission profile includes the wavelengths that cover the absorbance profile of the photoinitiators used in the CR.28
Conclusion
Based on the above results, we can conclude that a polymerization intensity <400 mW/cm2 leads to an increase in cytotoxicity levels and a significant decrease in the mechanical properties of composite resins.
We believe that this may go unnoticed by dentists in the short term. Therefore, we must emphasize the importance of monitoring the intensity of the light-curing units periodically. The lack of verifications, the use of insufficient intensities, and the application of inadequate protocols either in terms of application technique or application times will result in premature restoration failure and potentially irreversible biological consequences.
REFERENCES
1. Guerrero F M, Maya C CX, Vallejo L M. Evaluación del efecto citotóxico de una resina dental a base de siloranos sobre fibroblastos L929. Rev la Univ Ind Santander Salud. 2016 Jan 1;48(1):71-80. [ Links ]
2. Fujioka-Kobayashi M, Miron RJ, Lussi A, Gruber R, Ilie N, Price RB, et al. Effect of the degree of conversion of resin-based composites on cytotoxicity, cell attachment, and gene expression. Dent Mater. 2019 Aug 1;35(8):1173-93. [ Links ]
3. Cohn-Inostroza N. Efecto citotóxico de BisGMA en cultivos celulares de fibroblastos humanos mediante ensayos de MTT. CLAVES Odontol. 2015;22(74). [ Links ]
4. International Organization for Standardization. ISO 10993-5 Biological evaluation of medical devices - Part 5: Tests for cytotoxicity: in vitro methods. 2009. [ Links ]
5. Alzraikat H, Burrow M, Maghaireh G, Taha N. Nanofilled Resin Composite Properties and Clinical Performance: A Review. Oper Dent. 2018 Jul 1;43(4):E173-90. [ Links ]
6. International Organization for Standarization, ISO 4049: 2019, Dentistry - Polymer-based restorative materials. [ Links ]
7. Yap AU, Eweis AH, Yahya NA. Dynamic and Static Flexural Appraisal of Resin-based Composites: Comparison of the ISO and Mini-flexural Tests. Oper Dent. 2018;43(5):E223-31. [ Links ]
8. Bragança GF, Vianna AS, Neves FD, Price RB, Soares CJ. Effect of exposure time and moving the curing light on the degree of conversion and Knoop microhardness of light-cured resin cements. Dent Mater. 2020;36(11):e340-51. [ Links ]
9. Malkiewicz K, Wychowanski P, Olkowska-Truchanowicz J, Tykarska M, Czerwinski M, Wilczko M, et al. Uncompleted polymerization and cytotoxicity of dental restorative materials as potential health risk factors. Ann Agric Environ Med. 2017 Dec 23;24(4):618-23. [ Links ]
10. Selig D, Haenel T, Hausnerová B, Moeginger B, Labrie D, Sullivan B, et al. Examining exposure reciprocity in a resin based composite using high irradiance levels and real-time degree of conversion values. Dent Mater. 2015 May 1;31(5):583-93. [ Links ]
11. Aguiar TR, de Oliveira M, Arrais CAG, Ambrosano GMB, Rueggeberg F, Giannini M. The effect of photopolymerization on the degree of conversion, polymerization kinetic, biaxial flexure strength, and modulus of self-adhesive resin cements. J Prosthet Dent. 2015 Feb 1;113(2):128-34. [ Links ]
12. Chisini LA, Arangurem Karam S, Gioda Noronha T, Morello Sartori LR, Schmidt San Martin A, Demarco FF, et al. Platelet-Poor Plasma as a Supplement for Fibroblasts Cultured in Platelet-Rich Fibrin. Acta Stomatol Croat. 2017 Jun 15;51(2):133-40. [ Links ]
13. Romo-Huerta MJ, Cervantes-Urenda ADR, Velasco-Neri J, Torres-Bugarín O, Valdivia ADCM. Genotoxicity Associated with Residual Monomers in Restorative Dentistry: A Systematic Review. Oral Health Prev Dent. 2021;19(1):471-80 [ Links ]
14. Saramet V, Melescanu-Imre M, Tâncu AMC, Albu CC, Ripszky-Totan A, Pantea M. Molecular Interactions between Saliva and Dental Composites Resins: A Way Forward. Materials (Basel). 2021 May 13;14(10):2537 [ Links ]
15. Schubert A, Ziegler C, Bernhard A, Bürgers R, Miosge N. Cytotoxic effects to mouse and human gingival fibroblasts of a nanohybrid ormocer versus dimethacrylate-based composites. Clin Oral Investig. 2019 Jan 30;23(1):133-9. [ Links ]
16. Saikaew P, Phimolthares P, Phitakthanaakul P, Sirikul P, Mekrakseree S, Panpisut P. Effects of Color Modifier on Degree of Monomer Conversion, Biaxial Flexural Strength, Surface Microhardness, and Water Sorption/Solubility of Resin Composites. Polymers (Basel). 2021 Nov 11;13(22):3902. [ Links ]
17. Borges MG, Silva GR, Neves FT, Soares CJ, Faria-e-Silva AL, Carvalho RF, et al. Oxygen Inhibition of Surface Composites and Its Correlation with Degree of Conversion and Color Stability. Braz Dent J. 2021 Feb 2;32(1):91-7. [ Links ]
18. Pagano S, Lombardo G, Balloni S, Bodo M, Cianetti S, Barbati A, et al. Cytotoxicity of universal dental adhesive systems: Assessment in vitro assays on human gingival fibroblasts. Toxicol In Vitro. 2019 Oct;60:252-60. [ Links ]
19. Beltrami R, Colombo M, Rizzo K, Di Cristofaro A, Poggio C, Pietrocola G. Cytotoxicity of Different Composite Resins on Human Gingival Fibroblast Cell Lines. Biomimetics. 2021 Apr 20;6(2):26. [ Links ]
20. Gonçalves F, Campos LM de P, Rodrigues-Júnior EC, Costa FV, Marques PA, Francci CE, et al. A comparative study of bulk-fill composites: degree of conversion, post-gel shrinkage and cytotoxicity. Braz Oral Res. 2018 Mar 8;32(0). [ Links ]
21. Sadeghyar A, Watts DC, Schedle A. Limited reciprocity in curing efficiency of bulk-fill resin-composites. Dent Mater. 2020;36(8):997-1008. [ Links ]
22. Grazioli G, Francia A, Cuevas-Suárez CE, Zanchi CH, Moraes RR De. Simple and Low-Cost Thermal Treatments on Direct Resin Composites for Indirect Use. Braz Dent J. 2019 Jun 3;30(3):279-84. [ Links ]
23. Bin Nooh AN, Nahedh H Al, AlRefeai M, Alkhudhairy F. The Effect of Irradiance on the Degree of Conversion and Volumetric Polymerization Shrinkage of Different Bulk-Fill Resin-Based Composites: An In Vitro Study. Eur J Dent. 2021 May;15(2):312-9. [ Links ]
24. Demarco FF, Corrêa MB, Cenci MS, Moraes RR, Opdam NJM. Longevity of posterior composite restorations: Not only a matter of materials. Dent Mater. 2012 Jan;28(1):87-101. [ Links ]
25. Vaidya N, Kumar P, Pathak K, Punia SK, Choudhary A, Patnana AK. Comparative Evaluation of the Influence of Different Sports/Energy Drinks and Alcoholic Beverages on the Surface Roughness of Three Different Flowable Esthetic Restorative Materials: An In Vitro Analysis. J Int Soc Prev Community Dent. 2020;10(5):585-90. [ Links ]
26. Fonseca ASQS, Labruna Moreira AD, de Albuquerque PPAC, de Menezes LR, Pfeifer CS, Schneider LFJ. Effect of monomer type on the CC degree of conversion, water sorption and solubility, and color stability of model dental composites. Dent Mater. 2017;33(4):394-401. [ Links ]
27. Cazzaniga G, Ottobelli M, Ionescu AC, Paolone G, Gherlone E, Ferracane JL, et al. In vitro biofilm formation on resin-based composites after different finishing and polishing procedures. J Dent. 2017 Dec;67:43-52 [ Links ]
28. Par M, Repusic I, Skenderovic H, Tarle Z. Wavelength-dependent light transmittance in resin composites: practical implications for curing units with different emission spectra. Clin Oral Investig. 2019 Dec 10;23(12):4399-409. [ Links ]
Conflict of interest: The authors declare that they have no conflict of interest regarding the scientific information provided.
Funding note: This study was funded by the Student Research Support Program (PAIE) of the Sectoral Commission for Scientific Research (CSIC).
Note issued by the Ethics Committee: This study was approved by the Ethics Committee of the School of Dentistry. File No. 091900000154-18.
Received: March 11, 2022; Accepted: November 03, 2022










 texto em
texto em