Servicios Personalizados
Revista
Articulo
Links relacionados
Compartir
Odontoestomatología
versión impresa ISSN 0797-0374versión On-line ISSN 1688-9339
Odontoestomatología vol.23 no.38 Montevideo 2021 Epub 30-Sep-2021
https://doi.org/10.22592/ode2021n37e406
Case report
Conservative treatment of refractory medication-related osteonecrosis of the jaw using the PENTO protocol: A case report
1Unidad de Urgencia Odontológica, Centro de Urgencias de Ñuñoa, Servicio de Salud Metropolitano Oriente, Santiago, Chile. andresmelianrivas@yahoo.es
A 78-year-old patient seeks care for unresolved bleeding on the left maxillary alveolar ridge. He had a history of bone metastasis treated with IV zoledronic acid, which was discontinued after medical discharge and previous surgical treatment of osteonecrosis of the jaws in the affected site. Continuity disruption with inflammation at the bone ridge was observed, with blood, purulent, and necrotic exudate. The radiograph showed diffuse and osteolytic radiolucency of the affected area, and necrotic tissue was detected at a microscopic level. The affected area was rinsed with 0.12% chlorhexidine, 400 mg amoxicillin/clavulanic acid and pentoxifylline was administered every 12 hours, and tocopherol 1000 IU every 24 hours. The area was evaluated after a month. Antibiotic therapy was discontinued, and the patient continued to be monitored every two weeks. At six months, there is complete resolution, and mucosal and bone tissue healed without recurrence.
Keywords: Medication-Related Osteonecrosis of the Jaw; Bisphosphonates; Pentoxifylline; Tocopherol
Paciente de 78 años consulta por sangrado sin resolución, a nivel del reborde alveolar maxilar izquierdo. Con antecedentes de metástasis óseas tratadas con ácido zoledrónico endovenoso discontinuado por alta médica y tratamiento quirúrgico previo de osteonecrosis maxilar en sitio afectado. Se observó disrupción de continuidad con inflamación a nivel del reborde óseo, con salida de contenido hemático, purulento y necrótico. Radiográficamente se observó radiolucidez difusa y osteolítica del área afectada y tejido necrótico a nivel microscópico. Se realizó aseo del área afectada, enjuagues de clorhexidina 0,12%, administración de amoxicilina/acido clavulánico y de pentoxifilina 400 mg cada 12 horas y tocoferol 1000 UI cada 24 horas. Se evaluó al mes, donde se discontinua antibioterapia y se mantiene régimen establecido con controles cada 2 semanas. A los 6 meses se evidencia resolución completa, con cicatrización de la mucosa y del tejido óseo sin recidiva.
Palabras claves: Osteonecrosis de los maxilares asociada a medicamentos; Bifosfonatos; Pentoxifilina; Tocoferol
Paciente de 78 anos consultado por sangramento sem resolução, ao nível do rebordo alveolar superior esquerdo. Com história de metástases ósseas tratadas com ácido zoledrônico endovenoso descontinuado por alta médica e tratamento cirúrgico prévio de osteonecrose maxilar no local afetado. Foi observada interrupção da continuidade com inflamação ao nível da crista óssea, com extravasamento de sangue, conteúdo purulento e necrótico. Radiograficamente, radioluscência difusa e osteolítica da área afetada e tecido necrótico foram observadas ao nível microscópico. A área afetada foi limpa, enxágue com clorexidina 0,12%, amoxicilina / ácido clavulânico e 400 mg de pentoxifilina a cada 12 horas e 1000 UI de tocoferol a cada 24 horas. Foi avaliado em um mês, quando a antibioticoterapia é descontinuada e um regime estabelecido é mantido com controles a cada 2 semanas. Aos 6 meses, é evidenciada resolução completa, com cicatrização da mucosa e do tecido ósseo sem recorrência.
Palavras-chave: Osteonecrose da mandíbula associada a medicamentos; Bisfosfonatos; Pentoxifilina; Tocoferol
Introduction
Medication-related osteonecrosis of the jaw (MRONJ) is a debilitating condition in which patients exhibit progressive bone destruction in the maxilla and/or the mandible after exposure to antiresorptive and/or antiangiogenic medications.1 It is defined as the presence of exposed necrotic bone that can be probed through a fistula in the maxillofacial region and that persists for at least eight weeks, with no history of radiation therapy in the affected area.2 It is a complication related to bone-modifying agents commonly prescribed by multiple specialists (endocrinology, orthopedics, oncology), whose primary function is to inhibit osteoclast activity, resulting in decreased bone turnover. These agents have been used to treat several pathologies such as osteoporosis, multiple myeloma, Paget disease, and bone metastasis (from primary breast, prostate, and lung cancers).3 Although MRONJ is a rare condition, it can have a potentially severe impact on quality of life, affecting individuals in more advanced stages of the disease in particular.
The condition was first described in 2003 by Marx, who reported the first report correlating prolonged use of pamidronate and zoledronate with induced osteonecrosis of the jaw.1 After Marx’s initial report, other case reports appeared describing similar symptoms in patients treated with bisphosphonates. Therefore, this condition was named bisphosphonate-related osteonecrosis of the jaw. However, denosumab 4 and bevacizumab 5 were also associated with osteonecrosis of the jaw. This led to an increase in the prevalence of osteonecrosis cases, so the American Association of Oral and Maxillofacial Surgeons (AAOMS) in 2014 recommended changing the name from bisphosphonate-related osteonecrosis to medication-related osteonecrosis.3 The clinical presentations of MRONJ have been widely described in the scientific literature in the last 15 years.
Clinically, MRONJ can present as exposed and unexposed bone lesions, pain, infection, intraoral or extraoral fistula, pathological fracture, or hypoesthesia of the affected area.6 The etiology of this condition is not well understood and is currently under study. However, MRONJ is described as having multifactorial pathogenesis that involves the synergistic effect of trauma or infection and altered bone turnover in the context of exposure to antiresorptive drugs.1-6 Invasive dental procedures, including tooth extractions, implant placement, and periodontal surgery, are considered the main triggers for developing MRONJ (78% of cases), and only a small percentage of cases lack a clear cause. In addition, medical comorbidities, particularly those associated with wound healing (diabetes mellitus, smoking history, immunosuppressants), seem to play a role in MRONJ onset.1,6
The clinical management of MRONJ remains controversial, with no established guidelines. Various proposals and therapeutic approaches have been implemented with varying degrees of success. These include conventional conservative therapies with topical antiseptics and broad-spectrum antibiotics and more radical therapies, including extensive surgical procedures such as necrotic bone resection.7 The use of complementary therapies such as hyperbaric oxygen, laser therapy and surgical laser, platelet-rich plasma, recombinant human parathyroid hormone, and, in recent years, pentoxifylline and tocopherol (PENTO protocol) has also been proposed.6,7
This study aims to present a case of refractory MRONJ management after surgery using the PENTO protocol.
Background
The use of the PENTO protocol to treat mandibular osteoradionecrosis/ osteomyelitis and MRONJ has been described. Although these pathologies have different pathophysiological mechanisms, they are clinically similar to MRONJ and may present oral bone exposure that does not heal in eight weeks.8,9 This combination has been described as having a positive synergistic effect on these pathologies. However, its main action mechanism remains unclear.10
Pentoxifylline is a methylxanthine derivative used to treat peripheral arterial disease, ischemic heart disease, and intermittent claudication. It is a non-specific phosphodiesterase inhibitor that subsequently increases cAMP and adenosine triphosphate-5’ in erythrocytes, increasing their flexibility.11 Additionally, this drug decreases leukocyte adhesion to endothelial cells, increases prostacyclin production, and inhibits platelet aggregation, improving peripheral blood flow, reducing blood viscosity, and increasing the flexibility of red blood cell membranes, favoring microcirculation with increased tissue oxygenation.1,10,11 Tocopherol (vitamin E) is a class of organic chemical compounds with several methylated phenols. It reduces tissue fibrosis and free radical damage generated during oxidative stress, thus protecting cell membranes.12 Therefore, the synergistic use of pentoxifylline and tocopherol would positively favor endothelial function, thus promoting tissue healing, reducing local inflammation, and improving circulation to the affected necrotic areas.(1, 10-12)
Case description
A 78-year-old male patient seeks treatment at the Dental Service of the Ñuñoa Emergency Center, Santiago de Chile due to unresolved intraoral bleeding. The patient reports dull and radiating pain of mild intensity at the left maxilla, intermittent bleeding, and an evolution time of six months. The bleeding was triggered by food intake.
His history includes hypertension and prostate adenocarcinoma, the latter diagnosed and treated with radiation therapy (66 Gy) and prostatectomy in 2015 with a good response. He has been monitored since then. In 2017, he presented bone metastasis at the lumbar spine. He was treated with antiresorptive drugs: zoledronic acid in doses of 4 mg as a single intravenous infusion for 15 minutes every 28 days for 24 cycles. The treatment was discontinued because the patient was discharged in January 2019. In July of the same year, the patient underwent an emergency extraction of tooth 2.7 on account of pain. Two months after extraction, the patient presented with pain, increased volume, and bone tissue exposure. He sought care at the maxillofacial surgery service in his region, where he was diagnosed with MRONJ, treated with local sequestrectomy, and oral antibiotics with good response and healing. However, in January 2020, the patient reported continuous bleeding in the treated site after eating, which continued intermittently with pain in the area. This forced him to visit the closest emergency center. The patient was being treated with diethylstilbestrol, tramadol, amlodipine, and losartan. He reported no history of allergies, smoking, alcohol consumption, or use of exogenous substances.
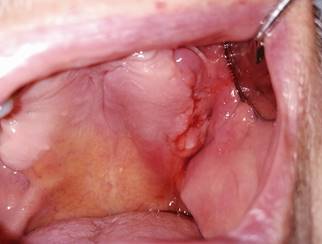
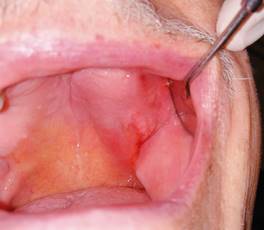
Fig. 2: Intraoral view of lesion on the left side of the maxilla with bleeding and inflammation of the surrounding tissues.
The first clinical examination found no evidence of facial asymmetries or volume increase (Fig. 1). The patient was completely edentulous and had a linear discontinuity at the mucosa of the left maxillary alveolar ridge of approximately 1 cm in diameter. It extended from the edentulous region of teeth 2.5-2.7 to the maxillary tuberosity. Clearly delimited lesion, with abundant bleeding and purulent exudate. Large edema and erythema of the surrounding soft tissues were also observed (Fig. 2). Communication with bone tissue was confirmed by probing the lesion and removing the remaining necrotic bone tissue and abundant hematic content (Fig. 3 A, B).
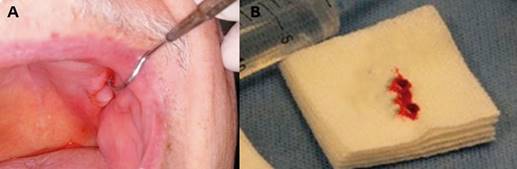
Fig. 3: (A) Intraoral view showing communication with the bone. (B) The remaining necrotic bone tissue was removed during probing.
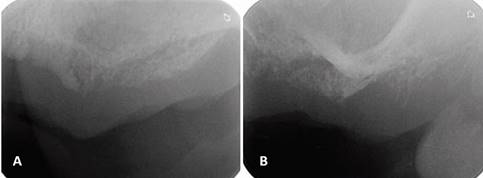
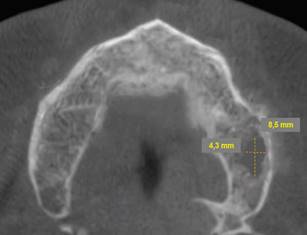
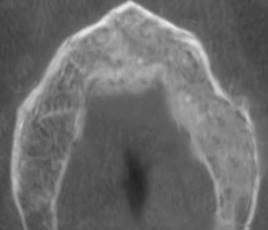
Periapical radiographs were initially ordered. They showed a loss of continuity of the bony trabeculae with irregular, faint osteolytic areas without root debris or foreign bodies (Fig.4 A, B). A CBCT of the maxilla was ordered to evaluate the extent of the lesion. The CBCT showed a radiolucent and osteolytic area at the maxillary bone tissue, measuring 8.5 mm x 5.4 mm, with irregular, poorly defined borders, and which did not involve the buccal, palatine, or maxillary sinus cortical areas (Fig. 5). Microscopic study of the necrotic tissue debris confirmed areas of bone tissue necrosis with inflammatory infiltrate, confirming the MRONJ diagnosis.
(A) Loss of bone density with a poorly defined radiolucent area. (B) Loss of bone trabeculation and of continuity of the bone ridge, with irregular radiolucent and radiopaque areas.
Cross-section showing an osteolytic area on the left side of the maxilla measuring 8.5 mm x 4.3 mm is observed.
Given the patient’s systemic and local history, conservative management was performed after the patient signed an informed consent. Twice-a-day rinsing with 0.12% chlorhexidine was prescribed for 14 days, as well as pentoxifylline 400 mg tablets every 12 hours and tocopherol 1000 IU, 1 tablet every 24 hours. Antibiotic treatment with amoxicillin/clavulanic acid (875/125 mg) was also indicated for 14 days to treat the co-occurring infection. The patient was monitored after 14 days. We observed a decrease in purulent content and inflammation, with persisting communication with bone tissue. Antibiotic treatment was discontinued after one month. The rest of the treatment remained unchanged (PENTO protocol + topical antiseptics). The patient would be monitored every two weeks. The clinical behavior of the lesion was evaluated every two weeks. The lesion was cleaned and irrigated with topical antiseptic 0.12% chlorhexidine. The patient reported improved symptoms after six months. He reported decreased pain and bleeding in the affected area. There was evidence of healing of the affected mucosa, continuity disruption, absence of bleeding, and no inflammation of the surrounding tissue (Fig. 6). A control CBCT was ordered, which showed bone neoformation, no radiolucent areas, and bone tissue without alterations (Fig. 7). The patient continued to be monitored for two months, with no clinical or radiographic signs of MRONJ recurrence.
Mucosal healing and decreased inflammation.
Bone neoformation and recovery of bone trabeculation with regression of radiolucent areas.
Discussion
Although there is consensus and recommended protocols, the evidence available is insufficient to develop standardized guidelines for the specific treatment at each stage of the disease. Therefore, MRONJ management remains controversial. In general, treatment aims to prevent and identify the disease early. Once the disease has been diagnosed, pain and infection should be controlled, bone necrosis progression should be minimized, and an adequate environment for tissue healing should be established.6 This is why less invasive and costly therapeutic alternatives are sought to treat MRONJ.
Four clinical studies have studied the therapeutic value of the pentoxifylline and tocopherol protocol in MRONJ treatment. In all cases, the PENTO protocol was implemented jointly with other treatments such as antibiotics, topical antiseptics, or minimal or no surgical intervention. This treatment relieved symptoms, healed the lesion and the bone to varying degrees.1,11 In 2010, Epstein et al. published the first case series in which pentoxifylline and tocopherol were used to treat bisphosphonate-associated osteonecrosis (BON). Six patients with BON were studied: four had a history of cancer, and two had a history of severe osteoporosis. They had been previously treated with bisphosphonates (five with IV bisphosphonates and one orally). They had all been treated with chlorhexidine rinses and antibiotics jointly with the PENTO protocol. The average prescription follow-up period was ten months. All six patients’ symptoms improved with the PENTO protocol. They had no pain, erythema, or purulence, in addition to a mean 74% reduction in the area of exposed bone. One case resolved, and only one patient required a sequestrectomy in the area of exposed bone. No case presented adverse effects, and all the patients showed good tolerance to the drugs.1,11,13 Also, in 2014, Magremanne and Reychler reported treating a 58-year-old male patient with PENTO protocol for stage-3 MRONJ. He had been treated with oral and intravenous bisphosphonates for corticosteroid-induced osteoporosis. Tooth extraction was the precipitating factor leading to ONJ. Two weeks after surgery, he developed pain at the extraction site. One month later, he developed lip paresthesia with soft tissue trauma from bone spicules. After nine months of multiple cycles of painkillers, antibiotics (clindamycin and doxycycline) and multiple curettages of the extraction site, the patient was treated with PENTO protocol. He was prescribed 12 months of the PENTO protocol and systemic antibiotics. Imaging studies showed bone recovery, bone healing, and symptom resolution, with no recurrence or adverse effects.1,11,14 In 2016, Owosho et al.9reported a case series including seven patients diagnosed with refractory MRONJ due to antiresorptive drugs for treating metastatic bone tumors and multiple myeloma (three patients with stage-3 MRONJ, three patients with stage-2 MRONJ, and one patient with stage-0 MRONJ). They were treated with the PENTO protocol for a mean period of 16.8 months, in addition to 0.12% chlorhexidine rinses and antibiotics. Patients’ mean age was 60.4 years, and they had a medical history of cancer metastasis (multiple myeloma, metastatic gastrointestinal stromal tumor, and metastatic breast cancer). Four patients had been treated with bisphosphonates, one with bisphosphonates associated with a tyrosine kinase inhibitor, one with bisphosphonates associated with monoclonal antibodies, and one was treated only with monoclonal antibodies. A sequestrectomy was performed in only two patients. Two patients experienced complete resolution of the exposed bone, two patients experienced partial resolution of the exposed bone, and one patient exhibited no change. Relief of painful symptoms was evidenced in all seven MRONJ patients, with significant bone neoformation observed at the final follow-up.1,11,12 Finally, in 2020 Seo et al.12 studied a case series involving nine patients with MRONJ treated with the PENTO protocol. The patients’ mean age was 76.1 years. Eight had suffered osteoporosis, and one had suffered multiple myeloma. All patients had been previously prescribed bisphosphonates, and most of them had tooth extractions as precipitating factors. None of the patients was treated with systemic antibiotics. The mean follow-up period of the PENTO protocol was 5.5 months, where eight patients underwent saucerization, and one of them underwent sequestrectomy for MRONJ treatment. Long-term prescription of the protocol (over three months) led to bone healing in all nine patients.1,9 These studies suggest that the synergistic use of these drugs can complement conservative and surgical MRONJ therapies with good results.
To date, there is no randomized clinical trial data regarding the use of pentoxifylline and tocopherol to treat MRONJ suggesting time and dose. However, clinical trials using the PENTO protocol to treat osteoradionecrosis of the jaw have reported optimal bone and mucosal healing with 800 mg of pentoxifylline/day and 1000 IU of tocopherol/day with average administration periods of six months.15 This coincides with the recommendations of other studies whose authors suggest considering the use of pentoxifylline and tocopherol for at least six months and then for as long as lesion regression is observed.8 Furthermore, all the studies that have implemented the PENTO protocol to treat MRONJ have used 400 mg of pentoxifylline doses every 12 hours and 400 IU of tocopherol also every 12 hours. They have had good results, no adverse reactions,1,11 and long average administration times ranging from 5.5 months (9) to 16.8 months.12 There are already proposals for its use in different stages of MRONJ. Foncea et al. 6 proposed an algorithm of prevention and treatment of MRONJ based on the stage of the disease. In stages 0 and 1, symptoms should be treated, and local factors should be managed, while in stages 2 and 3, medical surgical treatment should be provided based on the patient’s lesions. Therefore, the PENTO protocol should be used in every phase as adjuvant therapy: 400 mg of pentoxifylline with 400 mg of tocopherol twice a day, until symptom resolution.
Conclusion
The evolution of this case suggests that the synergistic use of pentoxifylline and tocopherol (PENTO protocol) is potentially useful to complement refractory MRONJ treatment; it is a well-tolerated therapy. It can alleviate symptoms and promote the healing of soft and bone tissue lesions, which was not achieved long-term with conventional conservative and surgical techniques. In any case, future clinical trials in larger cohorts would make it possible to evaluate its efficacy systematically and to determine its continuous and systematic use in MRONJ treatment protocols.
REFERENCES
1. Heifetz-Li JJ, Abdelsamie S, Campbell CB, Roth S, Fielding AF, Mulligan JP. Systematic review of the use of pentoxifylline and tocopherol for the treatment of medication-related osteonecrosis of the jaw. Oral Surg Oral Med Oral Pathol Oral Radiol. 2019;128(5):491-497.e2 [ Links ]
2. Govaerts D, Piccart F, Ockerman A, Coropciuc R, Politis C, Jacobs R. Adjuvant therapies for MRONJ: A systematic review. Bone. 2020;141(115676):115676. [ Links ]
3. Ruggiero SL, Dodson TB, Fantasia J, Goodday R, Aghaloo T, Mehrotra B, et al. American association of oral and maxillofacial surgeons position paper on medication-related osteonecrosis of the jaw-2014 update. J Oral Maxillofac Surg. 2014;72(10):1938-56. [ Links ]
4. Sivolella S, Lumachi F, Stellini E, Favero L. Denosumab and anti-angiogenetic drug-related osteonecrosis of the jaw: an uncommon but potentially severe disease. Anticancer Res. 2013;33(5):1793-7. [ Links ]
5. Pakosch D, Papadimas D, Munding J, Kawa D, Kriwalsky MS. Osteonecrosis of the mandible due to anti-angiogenic agent, bevacizumab. Oral Maxillofac Surg. 2013;17(4):303-6. [ Links ]
6. Foncea C, von Bischhoffshausen K, Teuber C, Ramírez H, Goñi I, Sánchez C, et al. Osteonecrosis of the jaws. Rev Med Chil. 2020;148(7):983-91 [ Links ]
7. Beth-Tasdogan NH, Mayer B, Hussein H, Zolk O. Interventions for managing medication-related osteonecrosis of the jaw. Cochrane Database Syst Rev. 2017;10:CD012432. [ Links ]
8. Martos-Fernández M, Saez-Barba M, López-López J, Estrugo-Devesa A, Balibrea-Del-Castillo JM, Bescós-Atín C. Pentoxifylline, tocopherol, and clodronate for the treatment of mandibular osteoradionecrosis: a systematic review. Oral Surg Oral Med Oral Pathol Oral Radiol. 2018;125(5):431-9. [ Links ]
9. Seo MH, Eo MY, Myoung H, Kim SM, Lee JH. The effects of pentoxifylline and tocopherol in jaw osteomyelitis. J Korean Assoc Oral Maxillofac Surg. 2020;46(1):19-27. [ Links ]
10. Lyons AJ, Brennan PA. Pentoxifylline - a review of its use in osteoradionecrosis. Br J Oral Maxillofac Surg. 2017;55(3):230-4. [ Links ]
11. Cavalcante RC, Tomasetti G. Pentoxifylline and tocopherol protocol to treat medication-related osteonecrosis of the jaw: A systematic literature review. J Craniomaxillofac Surg. 2020;48(11):1080-6. [ Links ]
12. Owosho AA, Estilo CL, Huryn JM, Yom SK. Pentoxifylline and tocopherol in the management of cancer patients with medication-related osteonecrosis of the jaw: an observational retrospective study of initial case series. Oral Surg Oral Med Oral Pathol Oral Radiol. 2016;122(4):455-9. [ Links ]
13. Epstein MS, Wicknick FW, Epstein JB, Berenson JR, Gorsky M. Management of bisphosphonate-associated osteonecrosis: pentoxifylline and tocopherol in addition to antimicrobial therapy. An initial case series. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;110(5):593-6. [ Links ]
14. Magremanne M, Reychler H. Pentoxifylline and tocopherol in the treatment of yearly zoledronic acid-related osteonecrosis of the jaw in a corticosteroid-induced osteoporosis. J Oral Maxillofac Surg. 2014;72(2):334-7. [ Links ]
15. Hayashi M, Pellecer M, Chung E, Sung E. The efficacy of pentoxifylline/tocopherol combination in the treatment of osteoradionecrosis: Pentoxifylline/tocopherol combination. Spec Care Dentist. 2015;35(6):268-71. [ Links ]
Authorship contribution: 1. Conception and design of study 2. Acquisition of data 3. Data analysis 4. Discussion of results 5. Drafting of the manuscript 6. Approval of the final version of the manuscript. ANMR has contributed in: 1, 2, 3, 4, 5, 6. JARD has contributed in: 3, 4, 5, 6.
Ethical issues: The information herein included has been approved and an informed consent has been signed.
Received: February 15, 2021; Accepted: May 09, 2021











 texto en
texto en