Services on Demand
Journal
Article
Related links
Share
Odontoestomatología
Print version ISSN 0797-0374On-line version ISSN 1688-9339
Odontoestomatología vol.23 no.38 Montevideo 2021 Epub Sep 30, 2021
https://doi.org/10.22592/ode2021n37e207
Research
Establishing and implementing a simplified protocol for the expansion and culture of human dental pulp stem cells (hDPSCs)
1Cátedra de Fisiología general y bucodental, Facultad de Odontología, Universidad de la República, Uruguay
2Cátedra de Materiales Dentales, Facultad de Odontología, Universidad de la República, Uruguay. ggrazioli@gmail.com
3 Área Terapia Celular y Medicina Regenerativa (ATCMR), Departamento Básico de Medicina, Hospital de Clínicas, Facultad de Medicina, Universidad de la República, Uruguay
4Cátedra de Histología y Embriología Bucodental, Facultad de Odontología, Universidad de la República, Uruguay
5Área Terapia Celular y Medicina Regenerativa (ATCMR), Departamento Básico de Medicina, Hospital de Clínicas, Facultad de Medicina, Universidad de la República, Uruguay
6Instituto Nacional de Donación y Trasplante (INDT), Ministerio de salud Pública- Hospital de Clínicas, Facultad de Medicina, Universidad de la República, Uruguay
Objectives:
To establish and implement a simplified protocol for the extraction, primary isolation, and culture of human dental pulp stem cells (hDPSCs). To analyze the isolated cells quantitatively and qualitatively.
Methodology:
Ten healthy third molars were donated by patients who attended the School of Dentistry, UdelaR, and gave their written consent. The teeth were processed within 48 hours. The teeth were sectioned to obtain the pulp tissue and processed with the explant method. Cell viability and marker expression were analyzed by flow cytometry at passages 4 and 12 and verified by immunocytochemistry.
Results:
The cells obtained had a vitality greater than 90% in all passages. We found the characteristic morphology and the expression of CD90, C105, CD73, CD29 and 166 mesenchymal stem cell markers by flow cytometry in both passages.
Conclusion:
It was possible to establish a cell isolation protocol that is highly successful and safe to isolate hDPSC.
Keywords: Adult Stem Cells; Dental Pulp; Cell Separation; Flow Cytometry; Mesenchymal Stem Cells
Objetivos:
Establecer e implementar un protocolo simplificado de extracción, aislamiento primario y cultivo de células madre derivadas de la pulpa dental humana (DPSCh). Analizar cuantitativamente y cualitativamente las células aisladas.
Metodología:
10 terceros molares sanos donados por pacientes que concurrieron a la Facultad de Odontología, UdelaR y otorgaron su consentimiento escrito fueron procesados antes de las 48 hs. Se realizó la fractura de la pieza para la obtención del tejido pulpar y se procesó por el método explante. Se analizó viabilidad celular y expresión de marcadores por citometría de flujo en pasajes 4 y 12 y se corroboró mediante inmunocitoquímica.
Resultados:
Las células obtenidas presentaron una vitalidad mayor al 90% en todos los pasajes, observándose una morfología característica y expresión de marcadores de células madre mesenquimales CD90, C105, CD73, CD29 y 166 mediante citometría de flujo en ambos pasajes.
Conclusiones:
Se logró establecer un protocolo de aislamiento y expansión celular, con alta tasa de éxito de una población de DPSCh.
Palabras clave: Células Madre Adultas; Pulpa Dental; Separación Celular; Citometría de Flujo; Células Madre Mesenquimatosas
Objetivos:
Estabelecer e implementar um protocolo simplificado para a extração, isolamento primário e cultura de células-tronco da polpa dentária humana (DPSCh). Analise as células isoladas quantitativa e qualitativamente.
Metodologia:
10 terceiros molares saudáveis doados por pacientes que frequentaram a Faculdade de Odontologia UdelaR e deram consentimento por escrito foram processados antes de 48 horas. A fratura da peça foi realizada para obtenção do tecido pulpar e processada pelo método do explante. A viabilidade celular e a expressão do marcador foram analisadas por citometría de fluxo nas passagens 4 e 12 e confirmadas por inmunocitoquímica.
Resultados:
As células obtidas apresentaram viabilidade superior a 90% em todas as passagens, observando uma morfologia característica e expressão dos marcadores de células-tronco mesenquimais CD90, C105, CD73, CD29 e 166 por citometría de fluxo em ambas as passagens.
Conclusões:
Foi possível estabelecer um protocolo de isolamento celular, com alta taxa de sucesso e segurança para isolar o DPSCh.
Palavras Chave: Células-Tronco Adultas; Polpa Dentária; Separação Celular; Citometría de Fluxo; Células-Tronco Mesenquimais
Introduction
Stem or stromal cells are undifferentiated cells that exist in the body in the embryonic, fetal, and adult stages. After differentiation, they give rise to specific cells that make up tissues and organs 1-3. According to their origin, they can be classified as embryonic stem cells, fetal stem cells, adult stem cells and induced pluripotent stem cells (iPSCs) 4. According to their differentiation potential, they can be classified as totipotent, pluripotent, multipotent, oligopotent, and unipotent 4.
Mesenchymal stromal cells (MSCs) are typically adherent to plastic under standard culture conditions, have self-renewal capacity, can differentiate to osteoblasts, adipocytes, and chondroblasts and express surface markers such as CD73, CD90 and CD105, and to a lesser degree, MHC-I, and negativity for MHC-II, CD11b, CD14, CD34, CD45 and CD31 5,6.
The concept of stem or stem cell from the population of cells with these characteristics has been widely questioned. However, it is recognized that there is a minority subpopulation with stem cell properties which we will refer to as MSCs 7,8.
Therefore, MSCs are considered promising for treating various diseases because of their availability, differentiation capacity, and immunomodulatory function 5,9,10.
Different "niches" for adult MSCs have been identified and described regarding teeth. These cells have different denominations depending on where they are located and they are designated with their acronym in English. To date, the following have been identified: Dental Pulp Stem Cells (hDPSCs), Stem Cells from Human Exfoliated Deciduous teeth (SHED), Periodontal Ligament Stem Cells (PDLSCs), Stem Cells from Apical Papilla (SCAPs) and Dental Follicular Stem Cells (DFSCs) 11-15.
These hDPSCs are a promising source of stem cells for regenerative medicine given their neural crest origin, which gives them particular characteristics compared to other mesenchymal stem cells 10,16-22. They are now recognized to have a high proliferative potential and remarkable plasticity, which encourages their isolation and clinical use 13,23,24. Various studies prove their capacity to differentiate into different cell lineages: fibroblastic, bone, adipose, chondral, nervous, muscular, and pancreatic lineages 10,16-22. Differences have even been observed in its behavior depending on the inflammatory state of the pulp, which must be considered when used 25,26.
Although hDPSCs were first isolated over 20 years ago 12, this procedure never been carried out in Uruguay. Given the significant variability of existing protocols 27, the challenge the procedure implies, and the scarce knowledge Uruguayan dentists have on cell culture, this study aimed to adapt the existing methods to establish our own protocol focused on simplicity, economy, and effectiveness to begin this line of research. This study was conducted in Uruguay for the first time using a simplified protocol of dental pulp extraction, primary isolation, culture, and cell expansion using healthy third molars extracted for orthodontic treatment. We aimed to qualitatively and quantitatively analyze the cells obtained until passage 12.
The null hypothesis of this work was not obtaining stem cells from human dental pulp.
Materials and methods
A) Selection, collection, and transport of dental specimens
The samples were obtained from patients of both sexes who attended the surgical services of the School of Dentistry, Universidad de la República, to have their third molars extracted for orthodontic reasons. They provided their informed consent to donate the teeth. This study was accepted by the Ethics Committee of the School of Dentistry - UdelaR, file number: 091900-000143-13
Healthy third molars were included in this study. This diagnosis was based on the consensus terminology of the American Association of Endodontists 28, regardless of their stage of eruption or apex development. However, they had to be intact after extraction. Patient age ranged from 16 to 30. The following teeth were excluded from the study: with any type of carious lesion, pulp inflammation, related infection, or need to section for extraction.
The sample included the healthy third molars of ten patients extracted for orthodontic treatment.
All the extractions were coordinated. A flask refrigerated at 4ºC with a transport medium was available during the surgery. This mixture of commercial culture medium Alpha-MEM (Capricorn Scientific, Germany) was supplemented with a high antibiotic load: 100 U/ml penicillin/streptomycin (Capricorn Scientific, Germany). This medium provided nutrients to the tooth cells after extraction and ensured their survival until they were processed in the laboratory. Additionally, the high antibiotic load inhibited bacterial growth that may have occurred during extraction.
Immediately after extraction, the teeth were placed in a transport medium and stored at 4ºC. They were transported with cold chain and processed within 48 hours after extraction at the Preclinical Laboratory of Tissue and Cellular Engineering of the National Institute of Donation and Transplants (LITYC - INDT).
This procedure was carried out in two stages: First, tooth preprocessing and then pulp tissue collection.
B) Tooth preprocessing
An acrylic cabinet where four hands can be inserted was made for this procedure. It made it possible to process the parts in a closed environment. This made it possible to reduce tooth and environmental contamination.
First, all the periodontal tissue remains were removed from the cabinet with a 14-15 Gracey curette (Figure 1a) to prevent sample contamination with cells other than those intended. A section line was then traced using an electric handpiece, and a carborundum disk was used to trace a groove towards the cement on the cementoenamel junction. To avoid overheating, which would be negative for the cells in question, enamel wear was avoided during groove tracing, and the work was done with four hands, maintaining constant irrigation with saline solution. The resulting groove signaled a section line. Under no circumstances was the pulp chamber exposed, ensuring the sterility of the pulp tissue (Fig. 1b and c). Once this procedure was completed, the specimen was returned to the transport medium for transport to the collection site.
C) Pulp tissue collection
The tissues were collected in a FORMA 1300 A2 Model 1386 laminar flow chamber (Thermo Scientific, Waltham, Massachusetts, USA). Before starting, the laminar flow cabinet was decontaminated with 70% alcohol and sterilized with UV light for 30 minutes.
The procedure was performed with four hands. Both the operator and the assistant wore sterile clothing, overtunic, cap, gloves, mask, glasses, and shoe covers.
The tooth was decontaminated with a gauze soaked in 70% alcohol before placing it into the laminar flow cabinet. Once inside, the tooth was sectioned in a controlled manner with two No. 101 forceps. The crown was taken with one forceps and the root with the other. Once the forceps were stable, various movements were performed: rotational movements in the opposite direction of both sectioned parts and breaking movements. This separated the anatomical crown from the roots (Fig. 1d).
Once the pulp tissue was exposed, it was collected with small dentin spoons and 15-25 endodontic files. All the tissue obtained was placed on a glass petri dish. The pulps obtained were washed with phosphate-buffered saline (PBS-GIBCO, Denmark).
D) Cell culture and expansion
Explant culture was performed, which entails collecting fragments of tissue-explants-from which the cells are intended to migrate. To do this, the pulp tissue was cut into blocks of less than 1 mm3 with two scalpel handles with 15C blades, thus creating the explants. A 6-well plate (Greiner CELLSTAR®, Sigma Aldrich, Missouri, USA) was taken. Four slots were created with the same scalpels by drawing a grid on the floor of each well, thus providing macro mechanical retention for explant fixation. One explant per groove intersection was attached (Fig. 1e).
Once the explants were fixed, each well of the culture plate was gently loaded and the liquid was placed from the walls to avoid displacing the explants. Three ml of clonogenic culture medium that was previously prepared with the following composition was placed: Alpha-MEM (Capricorn Scientific, Germany), 1% penicillin/streptomycin (Capricorn Scientific, Germany), 20% fetal bovine serum (Capricorn Scientific, Germany). It was kept at 4ºC and placed in a 37ºC bath before use to raise the temperature and prevent pulp tissue thermal stress. Finally, it was placed in an incubator (FORMA 311, Thermo Scientific, Waltham, Massachusetts, USA) at 37ºC and 5% CO2 in a humid environment.
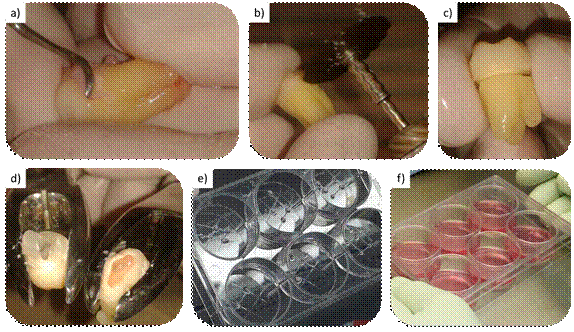
a) Cleaning periodontal debris; b and c) Filing for section line, without pulp exposure; d) Tooth sectioning using forceps. e) Explants placed in the grooves made for mechanical fixation; f) Placement of culture medium
Fig. 1: Step-by-step protocol for collecting and explanting hDPSCs
The culture medium was changed every three days. For each change, the old culture medium was gently aspirated with a 1000 μl micropipette (Finnpipette F2, Thermo Scientific, USA), and then 3 ml of clonogenic culture medium was added again.
The explant was kept in position until the desired cell expansion was achieved: 80% of the culture plate well covered with cells. Then, the cells underwent the first passage, and the explant was removed. Cell expansion occurred over approximately 15 days (Fig. 1f).
For each passage, we first gently removed the old culture medium with a 1000 μl micropipette and washed the well with the adhered cells by adding and removing 1 ml of PBS. Then, to isolate the cells, 1 ml of a commercial preparation of Trypsin/EDTA (TrypLE®, Thermo Scientific, Waltham, Massachusetts, USA) was placed in each well and then placed in the incubator at 37°C for 5 minutes. Afterwards, it was removed from the incubator, and the trypsin was neutralized by adding 2 ml of clonogenic culture medium to each well. In this way, a cell suspension was obtained in each well. They were then transferred to a t75 culture bottle (Greiner, Sigma Aldrich, Missouri, USA), and the explants were discarded.
Following this same cell expansion criterion (medium change every three days until reaching 80% confluency and then passaging), 12 passages were performed consecutively at ¼ of the cell suspension in each passage. The average confluency time in each passage was approximately seven days.
F) Evaluation of isolated cells
Viability and cell morphology by optical microscopy: we used an inverted microscope with phase contrast (Nikon Ti-S, USA), with fluorescence (Nikon, Ti-FL G-2A green 510-560 nm, USA), and a coupled camera (Nikon, DS-Fi1c-U2, USA).
Cell viability was assessed qualitatively at passages 1, 2, 4, 8, and 12 using a fluorescent viability indicator (Fluoroquench® One Lambda, Thermo Fisher, USA) at 5x magnification. For this, after passaging, 40 μl of cell suspension was placed in a well of Terasaki plate (Greiner, Sigma Aldrich, Missouri, United States), and 5 μl of Fluoroquench solution was added. It was left to rest for 15 minutes at room temperature and then observed under an inverted microscope with fluorescence, where viable cells were green and dead cells red.
Cell morphology was qualitatively evaluated in the different passages by phase contrast at 20x magnification. Image analysis was performed with NIS-Elements software (Nikon, USA).
Characterization by flow cytometry: In passages 4 and 12, cells were characterized with flow cytometry, with CD29, CD73, CD90, CD105 and CD166 as positive markers and CD34 and CD45 as negative markers (BD Biosciences, USA). Once 80% cell confluency was reached at passages 4 and 12, hDPSCs were resuspended in 1x phosphate buffered saline (PBS). The hDPSC suspension was incubated for 30 min at room temperature and without light with purified IgG monoclonal antibodies (specified above) labeled with fluorescein isothiocyanate (FITC), phycoerythrin (PE) or peridinin chlorophyll-protein complex with cyanine-5.5 (PerCP-Cy5.5). The cells were then washed with 1x PBS. Finally, the cells were analyzed with a flow cytometer (FACSCalibur™, BD Biosciences, USA).
Characterization by immunocytochemistry: An aliquot of cells was streaked on Millicell® plates (Millicell EZ SLIDE, Millipore, USA) at a concentration of 3000 cells per well as a double control procedure before performing passage 4 cytometric analysis. Cells were cultured for seven days, reaching high confluency, and plates were fixed in 10% formaldehyde. Primary markers CD90 and CD105 were used as positive markers and CD34 and CD45 as negative markers (Dako®, Denmark) for immunohistochemical characterization. Briefly, endogenous peroxidase was blocked using 0.9% hydrogen peroxide. The primary antibodies were incubated for 45 minutes and washed with PBS. The slides were then incubated with biotinylated antimouse/anti-rabbit antibodies and a streptavidin/peroxidase complex for 30 minutes each (LSAB þ-labeled streptavidin-biotin, Dako). A 3.30-diaminobenzidine-H20 substrate was used (Dako®, Denmark) to observe the reaction. Finally, the slides were counterstained with Mayer's hematoxylin solution. The primary antibodies were replaced with PBS as a negative control to immunocytochemistry.
As a final step, the cells obtained were frozen for future research. The slow freezing process was carried out by initially trypsinizing the culture bottles. The cell suspension was placed in 15 ml Falcon tubes and centrifuged at room temperature 400G (SORVALL ST-8R, Thermo Scientific, USA) for five minutes. Then, the supernatant was discarded, and the pellet was resuspended in 1 ml clonogenic medium where a Neubauer chamber was used for cell counting. The cells were diluted with the clonogenic medium until reaching a concentration of 1x106 cells/ml. The resulting cell suspension was placed in 2 ml cryotubes (Greiner, Sigma Aldrich, Missouri, United States), 1 ml was loaded into each cryotube, and 10% cryoprotectant (DMSO, Sigma Aldrich, Missouri, United States) was added. The cryotubes were quickly placed in a controlled cooling device (Nalgene® Mr. Frosty, Sigma Aldrich, Missouri, USA) with isopropyl alcohol directly into an ultrafreezer at -80 °C (FORMA 89000, Thermo Scientific, USA) for cryopreservation.
The rapid thawing method will be used in the future, where the cryovials will be removed from the ultrafreezer and placed directly in a bath at 37ºC until they become liquid. The cell suspension will immediately be placed in a Falcon tube with 10 ml of culture medium to dilute the effect of the cryoprotectant. The new cell suspension will be centrifuged at 400G for 5 minutes, the supernatant discarded, and the cells resuspended in clonogenic culture medium. Finally, the suspensions will be placed in t25 culture bottles for expansion, and the passage number will be recorded.
Statistical analysis: Viability analysis, cell morphology, and characterization by immunocytochemistry were qualitatively assessed. Flow cytometry results were analyzed with two-way ANOVA considering the a) marker used, b) passage analyzed (4 or 12). The data were previously subjected to a normality test, and the entire analysis was performed with a significance level of α=0.05. SigmaStat V3.5 software was used for this analysis.
Results
During the explant period, the first cells were observed to migrate from the explant one week after the start of the culture, resulting in an 80% confluency in the well after 15 days. Then, at each passage and for all the samples, 80% confluency in each bottle was achieved after approximately seven days.
Cell viability and morphology: High cell viability was observed in all passages. Fig. 2a and b show the fluorescence study representatively. The characteristic cell morphology of dental pulp mesenchymal stem cells can be observed in Fig. 2c and d.
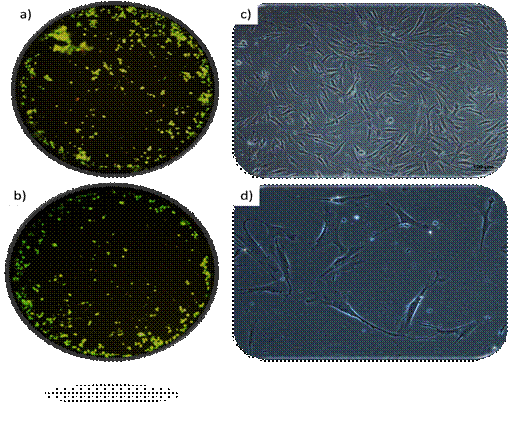
a) and b) Representative images of fluorescence cell viability assays (live green/dead red) at 40x magnification. c and d) Representative microphotographs of typical hDPSC morphology at 200x and 400x magnification, respectively
Fig. 2
Flow cytometry: the markers studied- CD29, CD73, CD90, CD105, and CD166- showed high expression (above 84%) in all cases in passages 4 and 12. Hematopoietic markers CD34 and CD45 showed low expression (below 10%) in both passages. The statistical analysis showed that when studying the time factor, although the expression of specific markers tended to decrease, no statistically significant differences were found in marker expression between passages 4 and 12 (p < 0.05). When comparing the specific markers, no statistically significant differences were found between passages 4 and 12. The same was true for hematopoietic markers. The expression values for the different markers are shown in Fig. 3.
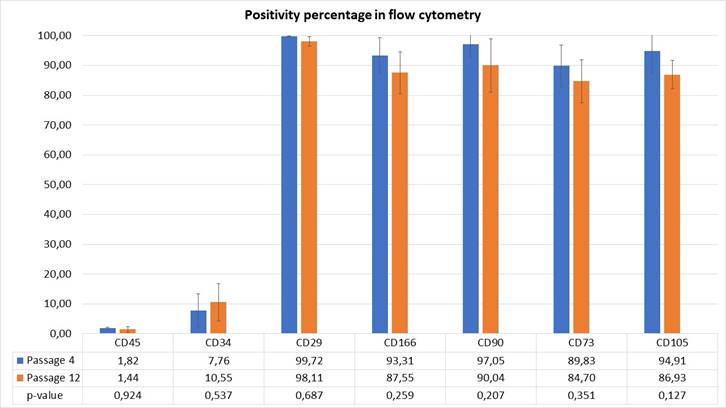
The p-value of the comparison in each marker for passages 4 and 12 is indicated
Fig. 3: Expression values (%) of hDPSCs cell markers
Immunocytochemistry: Immunocytochemistry confirmed the phenotypic profile of hDPSCs, showing positivity for CD90 and CD105 and no expression for CD34 and CD45, thus confirming the results of the flow cytometry. Fig. 4 shows marker expression by immunocytochemistry.

CD90 and CD105 show the characteristic pigmentation of the diaminobenzidine oxidation reaction; the absence of these pigments confirms the absence of CD34 and CD45 expression. 400x Magnification
Fig. 4: Microphotographs that represent the results of the immunocytochemical characterization of each marker
Discussion
This study aimed to establish a simplified protocol for the extraction, primary isolation, and culture of human dental pulp stem cells from healthy third molars that were extracted for orthodontic treatment. The results show that we can reject the null hypothesis since it was possible to obtain and characterize these cells.
Cell isolation from healthy teeth to be extracted, normally considered discarded biological material, third molars, first premolars and supernumerary teeth generally extracted for orthodontic treatment 29, is an attractive option because cell collection requires a minimally invasive technique (low morbidity of the donor site) and has the potential autologous use that entails immunocompatibility21,30,31.
The third molar is the last tooth to develop; therefore, it is in an early stage of development between the ages of 16 and 28. Studies have shown that non-carious extracted impacted third molars can produce an optimal amount of dental pulp tissue for hDPSC isolation 31,32. Therefore, we used third molars because they are the last to complete their development. This makes it possible to extract them even in incomplete development stages, providing them with a greater capacity to obtain pulp tissue volume and a better chance of finding cells with greater potency 31,32.
It has been possible to isolate DPSCs from healthy teeth and teeth with inflamed pulp33,34. As demonstrated by Inostroza et al.25, DPSCs isolated from inflamed dental pulp have a lower immunosuppressive capacity in vitro compared to those collected from healthy dental pulp, although they had the typical features of stem cells. However, Chen et al.26 found greater angiogenic potential in cells from inflamed pulps.
There is interest in isolating these cells because hDPSCs exhibit greater plasticity and proliferative potential than umbilical cord, bone marrow, and peripheral blood cells 21. The specific characteristics of teeth and their mineralized enamel provide an environment that is protected from external influences. This makes hDPSCs similar to embryonic cells despite their stage of development 21. The main advantage over embryonic stem cells is that hDPSCs do not create ethical controversies regarding their use. It is now possible to immortalize this cell lineage, which will allow us to broaden the field of application 16.
The versatility of hDPSCs has been proven in different in vivo studies in animal models with promising results for treating myocardial infarction, nerve tissue regeneration, muscular dystrophy, cerebral ischemia, corneal regeneration, and regarding their angiogenic capacity 10,16-22.
The literature includes two methods for hDPSC collection: enzymatic digestion of dental pulp and the explant method. Enzymatic digestion makes it possible to obtain cells quickly-in approximately 24 hours-although it requires specific reagents (collagenase and dispase). Releasing all the cells into the pulp tissue makes the population more heterogeneous and includes hDPSCs, fibroblasts, and even pericytes 35,36. On the other hand, migration in the explant method, based on cell migration in the culture outside the tissue, requires about 5 to 15 days. This results in a simpler, more economical method that provides a more homogeneous cell population 35,37,38. Also, as it does not require enzymes, it is simpler and more economical for its potential production for clinical use since enzymes certified for clinical use are expensive. This is why we selected the explant method for this study. The retention grooves created in the culture plate (Fig. 1e) are methodologically relevant because they facilitate explant adhesion. This is important because if the initial explant adhesion fails, the cells do not migrate out of the explant, and the sample is lost.
In this study, we implemented a pulp tissue extraction technique that reduces the risk of necrosis and contamination through working with four hands, constant irrigation with saline solution, and exposing the pulp tissue inside the chamber with two dental extraction forceps.
Regarding morphology (please see Fig. 2) hDPSCs have a characteristic fibroblastoid morphology, although they tend to show more than two branches 37,39. On the other hand, these images show cell homogeneity, typical of the explant method.
The isolation method proposed was effective for teeth stored for up to 48 h at 4ºC in transport medium. The outlook is promising since it provides a broader time window for processing and the possibility of creating a biobank. This is in agreement with the findings of Perry et al. 40, who obtained viable cells even within 120 hours.
As for cell viability, a high rate of live cells was obtained from passage 1 up to and including passage 12 as shown in Figure 2. These data confirm the study of Martín-Piedra et al. 41, who observed a high viability and proliferation rate in hDPSCs up to passage 11. As reaching passage 12 implies a prolonged culture time, the authors believe that the fact that this cell line does not undergo noticeable modifications until these passages is very relevant for future regenerative therapies.
These results can be confirmed in the literature as several studies have reported that hDPSCs maintain their cellular functionality, viability, doubling time at high passages (until passage 15) 35,39,41, even ensuring their correct cryopreservation, without significant changes in cell proliferation or differentiation capacity 42. However, although the effect of high passages on the expression of specific markers in other cell lines is known 43, this information is scarce and insufficiently studied for DPSCh 44.
The expression of specific markers and the negativity of hematopoietic markers in this study is in line with the data found in the literature 23,37,45. In this sense, this study provides relevant information, confirming that even up to passage 12, hDPSCs express specific markers with the same intensity without significantly increasing the expression of hematopoietic markers. These results confirm the successful collection of hDPSCs in our country and support the methodology and procedures applied as it was possible to maintain the homogeneity and expression of potentiality markers even up to passage 12.
According to Dominici et al. (2006) 46, these are the criteria for the characterization of mesenchymal stem cells: Fibroblastoid-like morphology, adherence to plastic, expression of surface markers CD73, CD90, CD105, CD11b, CD19, CD34, CD45, and HLA-DR, and differentiation to osteoblasts, adipocytes, and chondroblasts. These results should be interpreted with caution as we did not include trilineage differentiation assessment.
Future studies analyzing the ability to differentiate into different lineages will be necessary to demonstrate cellular functionality. Therefore, the results presented in this work provide scientific support for future research into regenerative therapies using tissue engineering.
Conclusions
It was possible to successfully establish a relatively simple protocol for the extraction, primary isolation, and culture of hDPSCs maintaining viability, cell morphology, and expression of specific cell surface markers even up to passage 12.
Although trilineage differentiation was not performed to complete cell characterization, the findings are encouraging, and future studies should verify this feature.
Further studies should focus on improving this procedure, emphasizing the need to create safer protocols that avoid xenogeneic products altogether and thus enable the safe use of these cells in regenerative therapies and tissue engineering.
Acknowledgments:
The authors would like to thank Prof. Dr. Ronell Bologna and the Laboratory of Molecular Pathology of the School of Dentistry, UdelaR, for their collaboration in the immunocytochemical analysis, to Dr. Hugo Godiño for his collaboration in the fieldwork, to Prof. Dr. Milka Bengochea for her contributions and all the INDT staff for helping with the logistics of this study.
REFERENCES
1. Kim T-W, Che J-H, Yun J-W. Use of stem cells as alternative methods to animal experimentation in predictive toxicology. Regul Toxicol Pharmacol. 2019 Jul;105(January):15-29 [ Links ]
2. Daley GQ. Stem cells and the evolving notion of cellular identity. Philos Trans B. 2015 Oct 19;370(1680):20140376. [ Links ]
3. Shyh-Chang N, Ng H-H. The metabolic programming of stem cells. Genes Dev. 2017 Feb 15;31(4):336-46 [ Links ]
4. Kolios G, Moodley Y. Introduction to Stem Cells and Regenerative Medicine. Respiration. 2013;85(1):3-10. [ Links ]
5. Li N, Hua J. Interactions between mesenchymal stem cells and the immune system. Cell Mol Life Sci. 2017 Jul 18;74(13):2345-60. [ Links ]
6. Mortada I, Mortada R, Al Bazzal M. Dental Pulp Stem Cells and Neurogenesis. In: Advs Exp Medicine, Biology-Neuroscience and respiration. 2017. p. 63-75. [ Links ]
7. Keating A. Mesenchymal Stromal Cells: New Directions. Cell Stem Cell. 2012 Jun;10(6):709-16. [ Links ]
8. Caplan AI. Mesenchymal Stem Cells: Time to Change the Name! Stem Cells Transl Med. 2017 Jun;6(6):1445-51. [ Links ]
9. Peng L, Ye L, Zhou X. Mesenchymal Stem Cells and Tooth Engineering. Int J Oral Sci. 2009 Mar;1(1):6-12. [ Links ]
10. Ji ST, Kim H, Yun J, Chung JS, Kwon S-M. Promising Therapeutic Strategies for Mesenchymal Stem Cell-Based Cardiovascular Regeneration: From Cell Priming to Tissue Engineering. Stem Cells Int. 2017;2017:1-13 [ Links ]
11. Honda MJ, Watanabe E, Mikami Y, Saito Y, Toriumi T, Shirakawa T, et al. Mesenchymal Dental Stem Cells for Tissue Regeneration. Int J Oral Maxillofac Implants. 2013 Jan;28(6):e451-60. [ Links ]
12. Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and invivo. Proc Natl Acad Sci. 2000 Dec 5;97(25):13625-30. [ Links ]
13. Ulmer FL, Winkel A, Kohorst P, Stiesch M. Stem cells--prospects in dentistry. Rev Mens suisse d'odonto-stomatologie 2010;120(10):860-83. [ Links ]
14. Sloan AJ, Waddington RJ. Dental pulp stem cells: what, where, how? Int J Paediatr Dent. 2009 Jan;19(1):61-70. [ Links ]
15. Honda MJ, Imaizumi M, Tsuchiya S, Morsczeck C. Dental follicle stem cells and tissue engineering. J Oral Sci. 2010 Dec;52(4):541-52. [ Links ]
16. Orimoto A, Kyakumoto S, Eitsuka T, Nakagawa K, Kiyono T, Fukuda T. Efficient immortalization of human dental pulp stem cells with expression of cell cycle regulators with the intact chromosomal condition. Papaccio G, editor. PLoS One. 2020 Mar 2;15(3):e0229996. [ Links ]
17. Luzuriaga J, Pastor-Alonso O, Encinas JM, Unda F, Ibarretxe G, Pineda JR. Human Dental Pulp Stem Cells Grown in Neurogenic Media Differentiate Into Endothelial Cells and Promote Neovasculogenesis in the Mouse Brain. Front Physiol. 2019 Mar 28;10(March):1-18. [ Links ]
18. Pai V, Vishwanath V, Prasanna J, Nadig R, Nadig R, Karthik J. Differentiation of isolated and characterized human dental pulp stem cells and stem cells from human exfoliated deciduous teeth: An in vitro study. J Conserv Dent. 2013;16(5):423. [ Links ]
19. Lan X, Sun Z, Chu C, Boltze J, Li S. Dental Pulp Stem Cells: An Attractive Alternative for Cell Therapy in Ischemic Stroke. Front Neurol. 2019 Aug 2;10(JUL):1-10. [ Links ]
20. Mead B, Logan A, Berry M, Leadbeater W, Scheven BA. Concise Review: Dental Pulp Stem Cells: A Novel Cell Therapy for Retinal and Central Nervous System Repair. Stem Cells. 2017 Jan;35(1):61-7. [ Links ]
21. Huang GT-J, Gronthos S, Shi S. Mesenchymal Stem Cells Derived from Dental Tissues vs . Those from Other Sources: Their Biology and Role in Regenerative Medicine. J Dent Res. 2009 Sep 18;88(9):792-806. [ Links ]
22. Longoni A, Utomo L, van Hooijdonk I, Bittermann G, Vetter V, Kruijt Spanjer E, et al. The chondrogenic differentiation potential of dental pulp stem cells. Eur Cells Mater. 2020 Feb 21;39:121-35. [ Links ]
23. Gronthos S, Brahim J, Li W, Fisher LW, Cherman N, Boyde A, et al. Stem Cell Properties of Human Dental Pulp Stem Cells. J Dent Res. 2002 Aug 13;81(8):531-5. [ Links ]
24. La Noce M, Paino F, Spina A, Naddeo P, Montella R, Desiderio V, et al. Dental pulp stem cells: State of the art and suggestions for a true translation of research into therapy. J Dent. 2014 Jul;42(7):761-8. [ Links ]
25. Inostroza C, Vega-Letter AM, Brizuela C, Castrillón L, Saint Jean N, Duran CM, et al. Mesenchymal Stem Cells Derived from Human Inflamed Dental Pulp Exhibit Impaired Immunomodulatory Capacity In Vitro. J Endod. 2020 Aug 1;46(8):1091-1098.e2. [ Links ]
26. Chen Y, Li X, Wu J, Lu W, Xu W, Wu B. Dental pulp stem cells from human teeth with deep caries displayed an enhanced angiogenesis potential in vitro. J Dent Sci. 2021 Jan 1;16(1):318-26. [ Links ]
27. Ferrúa CP, Centeno EGZ, Rosa LC da, Amaral CC do, Severo RF, Sarkis-Onofre R, et al. How has dental pulp stem cells isolation been conducted? A scoping review. Braz Oral Res. 2017 Dec 18;31:1-9. [ Links ]
28. Glickman GN. AAE Consensus Conference on Diagnostic Terminology: Background and Perspectives. J Endod. 2009 Dec;35(12):1619-20. [ Links ]
29. Yang X, Li L, Xiao L, Zhang D. Recycle the dental fairy's package: overview of dental pulp stem cells. Stem Cell Res Ther. 2018 Dec 13;9(1):347. [ Links ]
30. Scheller EL, Krebsbach PH, Konh DH. Tissue engineering: state of the art in oral rehabilitation. J Oral Rehabil. 2009 May;36(5):368-89. [ Links ]
31. Yalvac ME, Ramazanoglu M, Rizvanov AA, Sahin F, Bayrak OF, Salli U, et al. Isolation and characterization of stem cells derived from human third molar tooth germs of young adults: implications in neo-vascularization, osteo-, adipo- and neurogenesis. Pharmacogenomics J. 2010 Apr;10(2):105-13. [ Links ]
32. Atari M, Barajas M, Hernández-Alfaro F, Gil C, Fabregat M, Ferrés Padró E, et al. Isolation of pluripotent stem cells from human third molar dental pulp. Histol Histopathol. 2011;26(8):1057-70. [ Links ]
33. Pereira LO, Rubini MR, Silva JR, Oliveira DM, Silva ICR, Poças-Fonseca MJ, et al. Comparison of stem cell properties of cells isolated from normal and inflamed dental pulps. Int Endod J. 2012 Dec;45(12):1080-90. [ Links ]
34. Alongi DJ, Yamaza T, Song Y, Fouad AF, Romberg EE, Shi S, et al. Stem/progenitor cells from inflamed human dental pulp retain tissue regeneration potential. Regen Med. 2010 Jul;5(4):617-31. [ Links ]
35. Karamzadeh R, Eslaminejad MB, Aflatoonian R. Isolation, Characterization and Comparative Differentiation of Human Dental Pulp Stem Cells Derived from Permanent Teeth by Using Two Different Methods. J Vis Exp. 2012 Nov 24;69(69). [ Links ]
36. Catón J, Bostanci N, Remboutsika E, De Bari C, Mitsiadis TA. Future dentistry: cell therapy meets tooth and periodontal repair and regeneration. J Cell Mol Med. 2011 May;15(5):1054-65. [ Links ]
37. Hilkens P, Gervois P, Fanton Y, Vanormelingen J, Martens W, Struys T, et al. Effect of isolation methodology on stem cell properties and multilineage differentiation potential of human dental pulp stem cells. Cell Tissue Res. 2013 Jul 29;353(1):65-78. [ Links ]
38. Raoof M, Yaghoobi MM, Derakhshani A, Kamal-Abadi AM, Ebrahimi B, Abbasnejad M, et al. A modified efficient method for dental pulp stem cell isolation. Dent Res J (Isfahan). 2014 Mar;11(2):244-50. [ Links ]
39. Suchanek J, Soukup T, Visek B, Ivancakova R, Kucerova L, Mokry J. Dental pulp stem cells and their characterization. Biomed Pap. 2009 Mar 1;153(1):31-5. [ Links ]
40. Perry BC, Zhou D, Wu X, Yang F-C, Byers MA, Chu T-MG, et al. Collection, cryopreservation, and characterization of human dental pulp-derived mesenchymal stem cells for banking and clinical use. Tissue Eng Part C Methods. 2008 Jun;14(2):149-56 [ Links ]
41. Martin-Piedra MA, Garzon I, Oliveira AC, Alfonso-Rodriguez CA, Carriel V, Scionti G, et al. Cell viability and proliferation capability of long-term human dental pulp stem cell cultures. Cytotherapy. 2014 Feb;16(2):266-77. [ Links ]
42. Conde MCM, Chisini LA, Grazioli G, Francia A, Carvalho RV de, Alcázar JCB, et al. Does Cryopreservation Affect the Biological Properties of Stem Cells from Dental Tissues? A Systematic Review. Braz Dent J. 2016 Dec;27(6):633-40. [ Links ]
43. Wall ME, Bernacki SH, Loboa EG. Effects of Serial Passaging on the Adipogenic and Osteogenic Differentiation Potential of Adipose-Derived Human Mesenchymal Stem Cells. Tissue Eng. 2007 Jun;13(6):1291-8. [ Links ]
44. Ruparel NB, de Almeida JFA, Henry MA, Diogenes A. Characterization of a Stem Cell of Apical Papilla Cell Line: Effect of Passage on Cellular Phenotype. J Endod. 2013 Mar;39(3):357-63. [ Links ]
45. Vemuri MC, Chase LG, Rao MS. Mesenchymal Stem Cell Assays and Applications. In: Methods. Totowa, NJ: Humana Press; 2011. p. 3-8. (Methods in Molecular Biology; vol. 698) [ Links ]
46. Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F., Krause DS, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006 Aug;8(4):315-7. [ Links ]
Authorship contribution: 1. Conception and design of study 2. Acquisition of data 3. Data analysis 4. Discussion of results 5. Drafting of the manuscript 6. Approval of the final version of the manuscript. AF has participated in: 1,2,3,4,5,6. GG has participated in: 1,2,3,4,5,6. LE has participated in: 2,3,4,5,6. AM has participated in: 1,4,6. CT has participated in: 3,4,6. IA has participated in: 1,3,4,5,6.
Conflict of interest: The authors declare that they have no conflict of interest regarding the scientific information provided.
Ethics Committee: This study was approved by the Ethics Committee of the School of Dentistry. File No. 091900-000143-13.
Received: July 07, 2020; Accepted: May 27, 2021











 text in
text in 



