Serviços Personalizados
Journal
Artigo
Links relacionados
Compartilhar
Odontoestomatología
versão impressa ISSN 0797-0374versão On-line ISSN 1688-9339
Odontoestomatología vol.23 no.37 Montevideo 2021 Epub 30-Abr-2021
https://doi.org/10.22592/ode2021n37a9
Reporte de caso
Use of propolis with calcium hydroxide as an intracanal medicament in asymptomatic apical periodontitis. A case report
11 Posgrado Endodoncia, Universidad Tecnológica de México -UNITEC MÉXICO- Campus Odontología. México. eecarlosandrade@gmail.com
Various civilizations have used honey and its products for therapeutic purposes throughout history because of their healing effects. There is a renewed interest in the use of apitherapy as an adjunct in various medical treatments. Propolis stands out for its bactericidal, anti-inflammatory, and anti-cancer properties, among others. This paper aims to describe the case of a lower left first molar diagnosed with pulp necrosis and asymptomatic periapical periodontitis that was managed with a mixture of propolis and calcium hydroxide as an intracanal medicament between appointments. A bone repair process was observed during this period. It is concluded that propolis is a viable alternative as a calcium hydroxide adjuvant when intracanal medication is indicated.
Keywords: propolis; intracanal medicament; root canal treatment; successful; calcium hydroxide
La miel y sus productos, a lo largo de la historia, han sido usados por diversas civilizaciones con fines terapéuticos debido a sus efectos curativos. En la actualidad existe un renovado interés en el uso de la apiterapia como coadyuvante en diversos tratamientos médicos, en donde destaca el propóleo por sus propiedades bactericidas, antiinflamatorias, anticancerígenas, entre otras. El objetivo de este artículo es describir un caso de un primer molar inferior izquierdo con diagnóstico de necrosis pulpar y periodontitis periapical asintomática que fue manejado con el uso de la mezcla de propóleo con hidróxido de calcio como medicación intraconducto entre citas, logrando observar un proceso de reparación ósea durante este lapso. Se concluye que el propóleo es una alternativa viable como coadyuvante del hidróxido de calcio en los casos que está indicada la medicación intraconducto.
Palabras clave: Propóleo; medicamento intraconducto; Tratamiento de conductos radiculares; éxito; hidróxido de calcio
O mel e seus produtos, ao longo da história, têm sido utilizados por várias civilizações para fins terapêuticos devido aos seus efeitos curativos. Atualmente, há um interesse renovado no uso da apiterapia como adjuvante em vários tratamentos médicos, onde a própolis se destaca por seu bactericida, antiinflamatório, anticâncer, entre outros. O objetivo deste artigo é descrever um caso de primeiro molar inferior esquerdo com diagnóstico de necrose pulpar e periodontite periapical assintomática que foi gerenciada com o uso da mistura de própolis com hidróxido de cálcio como medicamento intraconducto entre as consultas, conseguindo observar um processo de reparo ósseo durante esse período. Em conclusão, a própolis é uma alternativa viável como adjuvante ao hidróxido de cálcio nos casos indicados por medicação intraconducto.
Palavras-chave: Própolis; medicação intraconducto; tratamento do canal radicular; sucesso; hidróxido de cálcio
Introduction
The literature has widely reported that the appearance, development, and persistence of pulp and periapical pathology is mainly caused by bacterial factors1-2. This connection between bacterial infection and periapical inflammation was established in Kakehashi’s classic study3, where an animal model showed that periapical lesions did not develop in pulp exposures in the absence of microorganisms. On the contrary, tissue repair occurred. However, tissue necrosis and periapical lesion occurred in pulps exposed in the presence of microorganisms. When bacteria lead to pulp inflammation, and there is no early treatment, inflammation spreads and can lead to necrosis over a variable period of time. Bacteria and their components reach the periodontium through the apical orifice or accessory canals, causing periodontitis4.
Periapical inflammation is a defense barrier against bacteria, as it aims to keep them inside the canal. Initially, there is chronic inflammatory infiltrate around the apical orifice that can be seen histologically. There appear osteoclasts that initiate bone resorption stimulated by mediators such as interleukin-1 (IL-1), tumor necrosis factor (TNF), and prostaglandins (PGE2)2,4.
The main aim of canal treatment in asymptomatic apical periodontitis is to decrease the microbial load in the root canal system through adequate chemo-mechanical cleaning and formation and canal obturation. This creates the necessary conditions to favor periapical tissue repair5-6. Therefore, it is essential to use an intracanal medicament to aid this disinfection and detoxification of the canal system.
Background
Hermann introduced paste calcium hydroxide in 1920 as an endodontic antimicrobial drug7-8. Since then, it has been widely used in different clinical settings9. Its clinical applications in endodontics include its use as an antimicrobial agent, an anti-inflammatory substance, and to induce hard tissue formation, control root canal exudation, and as a component of some endodontic sealants, among other uses10.
The mechanism of action of calcium hydroxide is directly attributed to its ability to dissociate into calcium and hydroxyl ions. Tronstad showed that the pH of necrotic teeth ranged from 6 to 7.4. After treatment with Ca(OH)2, their pH increased to a range of 7.4 - 9.6 in the dentin farthest from the main canal and to 8 - 11.1 in the dentin closest to the medication. This shows that the application of calcium hydroxide creates a more alkaline environment11.
Its antibacterial action occurs mainly through hydroxyl ions, as they rupture the membrane of bacteria, leading to bacterial death. Additionally, creating a highly alkaline environment also contributes to this action11. Hydroxyl ions must be able to diffuse through dentin consistently to achieve antibacterial action effectively12.
Several substances (anesthetic, hypochlorite, glycerine, etc.) have been added to calcium hydroxide to improve its properties: antibacterial action, fluidity, handling, etc. The ideal vehicle should enable the gradual and constant release of calcium and hydroxyl ions. Suggested vehicles can be classified as aqueous or viscous; aqueous vehicles promote faster ion dissolution, creating quicker but less durable antibacterial action, while viscous vehicles release ions more slowly but for longer13.
Hence the need to find a substance that can be mixed with calcium hydroxide and that creates synergy, thus harnessing the effects of the medication. In this context, the scientific community has turned to alternative medicine in search of elements that adapt to endodontic therapy. Therefore, the use of propolis as a calcium hydroxide vehicle becomes of great interest given its potential beneficial effect7,14-16.
The use of bee honey derivatives for medicinal purposes has been widely reported throughout history7-8,10,14. Propolis is one of them: a substance made by bees and used by human groups since ancient times to treat superficial wounds, burns, gastric and respiratory diseases7-9. Its use as medicine started empirically, and it was effective without people being sure of its therapeutic properties10.
There are reports in the literature that show its effectiveness against the following microorganisms: Enterococcus faecalis, Peptostreptococcus, Lactobacillus acidophilus, Actinomyces naeslundii, Prevotella oralis, Prevotella melaninogenica, Porphyromonas gingivalis, Fusobacterium nucleatum and Veillonella parvula. This action is mainly explained through the interaction of propolis ions with bacterial membranes: propolis disorganizes them and leads to cell death7,10,14,17,).
The current literature has found evidence that including propolis as a mouthwash ingredient helps reduce the number of bacterial colonies in dental plaque, with fewer cytotoxic effects for fibroblasts than chlorhexidine. It has also been used with satisfactory results to treat canker sores and as an adjuvant in healing post-surgical wounds18.
Regarding the use of propolis in various endodontic treatments, it has shown effectiveness in pulp coating, pulpotomies, apexogenesis, irrigation protocols, and as an intracanal medicament19.
Case report
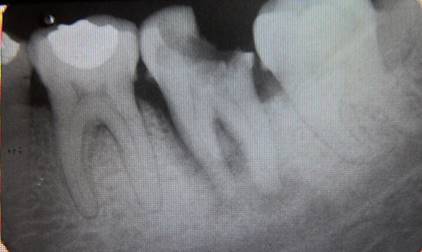
A 40-year-old female patient who attends the endodontics clinic after being referred by her health center to assess and treat tooth 37, which presents a deep carious lesion. Her dental history was taken, and an oral exam was performed. Pulpal sensitivity tests, performed with tetrafluoroethane (Endo-Ice, Coltene Whaledent, USA 2010), as well as periapical sensitivity tests, were negative. A 5-mm gingival sulcus was found on probing in the mesial area, as well as an absence of tooth mobility and surrounding soft tissue without inflammation. The radiographic study (Fig. 1) showed crown destruction on the occlusal and distal sides, pulp chamber communicating with the oral cavity, radiolucent lesion associated with apex of the distal root, and peripheral radiolucent lesion in mesial root including furcation area. The data were collected and analyzed, leading to the diagnosis of pulp necrosis with asymptomatic apical periodontitis.
Periapical lesion affecting both roots and furcation area involvement
The treatment plan was then established: root canal treatment with intracanal medication replacement. Calcium hydroxide with propolis is used as an intracanal medicament. The patient was informed of this procedure and gave her consent for the protocol.
The inferior dental nerve was anesthetized with lidocaine with vasoconstrictor at 1:100,000. After absolute isolation was in place, the access cavity was made with a #4 round bur and ultrasound tips (Varios 3500 NSK, 2010 Japan). The tartar found in the pulp chamber was removed. Then, the root was accessed with Gates-Glidden burs, the working length of the three canals was determined with a foramen locator (MiniApex Sybron Endo, USA 2013) and subsequently confirmed radiographically. The canals were instrumented with Mtwo rotary files (VDW, USA 2012): mesial canals with a #40.06 file and distal canals with a #50.04 file. The irrigation protocol was specifically designed for this study since there was no reference in the literature. It was created with the help of two lecturers of the Postgraduate Degree in Endodontics. During instrumentation, 10 ml of saline solution was used to remove the debris and lubricate the instruments. The final irrigation was performed with 3 ml of EDTA to remove the smear layer and promote greater intracanal medicament diffusion. Sodium hypochlorite was not used to allow the intracanal medicament of propolis with calcium hydroxide to act as an antibacterial agent. The canals were dried, and the propolis (20% Gourmiel bee propolis, authorized, produced, and distributed under Mexican official standard NOM-003SAG/GAN-2017) mixture with calcium hydroxide at a 2:1 ratio was prepared (Fig. 2). Once the preparation was homogeneous, it was placed inside the canals with a final apical file. PTFE and Cavit were placed as a temporary restoration.
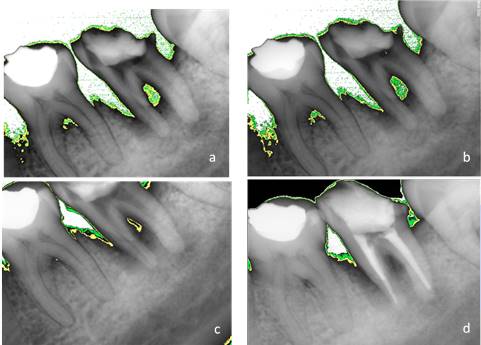
The intracanal medicament was replaced after 7, 15, and 21 days. An X-ray was taken at each appointment. Additionally, an analysis was conducted with Kodak software. The areas of periradicular bone density were digitally assessed at each appointment: white showed absence, green 2.5%, and yellow 7%.
Finally, after 28 days, the patient being asymptomatic and with no intracanal exudation, the practitioner decided to seal the canal system with gutta-percha with thermoplastic AH-Plus sealant. A final radiography is taken and compared to previous images (Fig. 3). The intermediate restoration was made with IRM. The patient was referred to the Dental Prosthesis Department of the School of Dentistry for crown rehabilitation.
Discussion
Several authors throughout history have supported the use of calcium hydroxide as an intracanal medicament1-2,11-13,20-21 since the benefits are clear. First, it has an alkaline pH that has a negative effect on bacterial population developments. It also releases hydroxyl ions acting as bactericidal agents. It seals the canals temporarily, thus preventing nutrient filtration for pathogenic microorganisms to some extent. It also has tissue compatibility characteristics.
Regarding its antibacterial effect, Pimenta et al.22 designed a study comparing various antibacterial paste compositions, including the mixture of propolis and calcium hydroxide, calcium hydroxide alone, and propolis alone. The efficacy of propolis against Enterococcus faecalis was similar with or without the calcium hydroxide mixture, successfully inhibiting colony growth. Victorino et al. conducted an in vitro study 23 where they created two propolis-based kinds of toothpaste and compared them with a calcium hydroxide paste to assess their antibacterial efficacy against strains of Staphylococcus aureus, Kocuria rhizophila, Pseudomona aeruginosa, Streptococcus mutans and Escherichia coli. Propolis-based toothpastes were superior to calcium hydroxide against these bacteria.
Propolis has also been successfully used as an endodontic irrigant. Jaiswal et al. 24 compared propolis, sodium hypochlorite, and chlorhexidine against Enterococcus faecalis biofilms in vitro. The propolis group showed no statistically significant differences compared to sodium hypochlorite. Therefore, they conclude that the propolis irrigant solution could be an alternative to sodium hypochlorite without its toxic effects. Kalyoncuoglu et al. conducted an interesting study on resins adherence to dentin 25. They found that a final irrigation protocol with a 20% propolis solution had better effects on the adhesion strength of self-etching adhesives than other irrigants.
Rezende’s in vitro study15 was among the first to include propolis as a component of the intracanal medication. This led to the mixture of propolis with calcium hydroxide being highly efficient in controlling odontogenic infections. In 2019, El-Tayeb and Abu-Sheida26 conducted an in vivo experiment in dogs to evaluate propolis’ antibacterial and regenerative activity in immature necrotic teeth. They concluded that propolis has similar characteristics to triantibiotic paste so that it can be used as an alternative in revascularization therapies.
In turn, Shabbir et al.27 conducted an in vivo study where propolis was used as an intracanal medicament in 80 patients, assessing its effect on postoperative pain. The results show that the use of propolis in necrotic teeth effectively prevents postoperative pain, so they recommend that this compound be used as an intracanal medicament between appointments. Given the above, for this study, we decided to apply the experimental model to in vivo use, as we knew the drug had been used successfully used in previous studies.
Propolis has been widely used by societies since ancient times, albeit empirically. However, we currently have scientific evidence supporting the fact that it is safe for humans to consume as food and as a medicinal agent. The only known contraindication is its application in patients allergic to bee products. This and its significant therapeutic benefits led us to integrate this substance to manage this case, with the certainty that it did not pose a risk for the patient7,10,14.
During the treatment, the radiographic evaluations showed progressive bone apposition and, therefore, a process of repair of the periapical lesion. This is consistent with Toker’s findings28 in a study with rats with apical periodontitis: an increase in bone density was observed after the use of propolis. Pilegi’s in vitro study29 also provides evidence that propolis inhibits osteoclast maturation by promoting lesion repair.
Although radiographies provide interesting data, a histological study would be beneficial to evaluate the injury-repair process further, so this type of methodology is suggested as a future line of research.
The treatment cannot be considered successful yet as more control appointments are needed to evaluate the repair process. However, progress is observed in the repair process of the periapical lesion.
This case report cannot establish that propolis as a calcium hydroxide vehicle that will promote a better prognosis for this type of pathology. However, it aims to make the scientific community interested in expanding this type of research-especially in vivo studies-since there is currently enough supporting laboratory research.
REFERENCES
1. Cohen S, Burns R. Vías de la pulpa. 10ª edición. Barcelona: Elsevier, 2011. 1004 pp. [ Links ]
2. Ørstavik D, Ford P. Essential Endodontics. London: Blackwell Science; 1999. 410 pp. [ Links ]
3. Kakehashi S, Stanley HR, Fitzgerald RJ. The effects of surgical exposures of dental pulps in germ-free and conventional laboratory rats. Oral Surg Oral Med Oral Pathol 1965; 20: 340-349. [ Links ]
4. Pumarola J, Canalda C. Endodoncia. 3ra ed. Barcelona: Masson; 2001. 367 pp. [ Links ]
5. Nair PNR. Pathogenesis of apical periodontitis and the causes of endodontic failures. Crit Rev Oral Biol Med. 2004; 15(6): 348-381. [ Links ]
6. Kawashima N, Wadachi R, Suda H, Yeng T, Parashos P. Root canal medicaments. Int Dent J. 2009; 59(1): 5-11. [ Links ]
7. Parolia A, Thomas S, Kundabala M, Mohan M. Propolis and its potential uses in oral health. Int J Med and Medic Sci. 2010; 2(7): 210-215. [ Links ]
8. Lavandera I. Curación de heridas sépticas con miel de abejas. Rev Cubana Cir. 2011; 50(2): 187-196. [ Links ]
9. Cabrera L, Rodriguez G. Actividad antibacteriana de miel de abejas multiflorales de cuatro zonas apícolas del estado Zulia, Venezuela. Rev Científ FCV-LUZ. 2003; 3(8): 205-211. [ Links ]
10. Marcucci M. Propolis: chemical composition, biological properties and therapeutic activity. Apidolog. 1995; 28: 83-99. [ Links ]
11. Tronstad L, Andreasen JO, Hasselgren G, Kristerson L, Riis I. Ph changes in dental tissues after root canal filling with calcium. J Endod 1981; 7(1): 17-21. [ Links ]
12. Esberard M. Changes in pH at the Dentin Surface in Roots Obturated with Calcium Hydroxide Pastes. J Endod 1996; 22(8):134-38. [ Links ]
13. Andrade F, Almeida P. Evaluation of pH levels and calcium ion release in various calcium hydroxide endodontic dressings. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004; 97: 388-92. [ Links ]
14. Ahuja V, Ahuja A. Apitherapy - A sweet approach to dental diseases. Part II: Propolis. J. Academy Adv Dental Research. 2011; 2(2): 1-7. [ Links ]
15. Rezende G, Acosta L, Baroni D. In vitro antimicrobial activity of endodontic pastes with propolis extracts and calcium hydroxide. Braz Dent J 2008; 19(4): 301-305. [ Links ]
16. Victorino F, Franco S. Pharmacological evaluation of propolis solutions for endodontic use. Pharma Biol. 2007; 45(9): 721-727. [ Links ]
17. Koru O, Toksoy F. In a vitro antimicrobial activity of propolis samples from different geographical origins against certain oral pathogens. Anaerobe. 2007; 13:140-145. [ Links ]
18. Wieckiewicz W, Miernik M, Wieckiewicz M, and Morawiec T. Does propolis help to maintain Oral health? Evid Based Complement Alternat Med. 2013:1-8. [ Links ]
19. Ahangari Z, Naseri M. Propolis: Chemical composition and its applications in endodontics. Iran Endod J. 2018; 13(3):285-292. [ Links ]
20. Estrela C, Pécora JD, Souza-Neto MD, Estrela CRA, Bammann LL. Effect of vehicle on antimicrobial properties of calcium hydroxide pastes. Braz Dent J. 1999; 10: 63-72. [ Links ]
21. Sathorn C, Parashos P, Messer H. Antibacterial efficacy of calcium hydroxide intracanal dressing: a systematic review and meta-analysis. Int Endod J 2007; 40: 2-10. [ Links ]
22. Pimenta H, Violante I, Musis C, Borges A, Aranha A. In vitro effectiveness of Brazilian brown propolis against Enterococcus faecalis. Braz Oral Res. 2015; 29(1): 1-6. [ Links ]
23. Victorino R, Bramante C, Watanabe E, Ito I, Franco S, Hidalgo M. Antibacterial activity of propolis-based toothpastes for endodontic treatment. Braz. J. Pharm. Sci. 2009; 45(4): 795-800. [ Links ]
24. Jaiswal N, Dakshita S, Udai S, Kanwardeep S, Urja A, Shivika G. Evaluation of antibacterial efficacy of Chitosan, Clorhexidine, Propolis and Sodium hypoclorite on Enterococcus faecalis biofilm: an in vitro study. J Clin Exp Dent. 2017; 9(9): 1066-74. [ Links ]
25. Kalyoncuoglu E, Gönülol N, Özsezer E, Bodrumlu E. Effect of Propolis as a root canal irrigant on bond strength to dentin. J Appl Biomater Funct Mater. 2015; 13(4):362-366. [ Links ]
26. El-Tayeb M, Abu-Seida A, El Ashry S, El-Hady S.. Evaluation of antibacterial activity of propolis on regenerative potential of necrotic immature permanent teeth in dogs. BMC Oral Health. 2019; 19(174): 1-12. 27 Shabbir J, Qazi F, Farooqui W, Ahmed S. Effect of propolis paste as intracanal medicament on post-endodontic pain: a double blind randomized clinical trial. Int J Environ Res Public Health. 2020; 17(2): 445. [ Links ]
27 Shabbir J, Qazi F, Farooqui W, Ahmed S. Effect of propolis paste as intracanal medicament on post-endodontic pain: a double blind randomized clinical trial. Int J Environ Res Public Health. 2020; 17(2): 445 [ Links ]
28. Toker H, Ozan F, Ozer H, Ozdemir H. A morphometric and histopathologic evaluation of the effects of propolis on alveolar bone loss in experimental periodontitis in rats. J Periodontol. 2008; 79(6): 1089-94. [ Links ]
29. Pileggi R, Antony K, Johnson K. Propolis inhibits osteoclast maturation. Dent Traumat. 2009; 25(6): 584-588. [ Links ]
Authors' contribution note: 1.Conception and design of study 2.Acquisition of data 3.Data analysis 4.Discussion of results 5.Drafting of the manuscript 6.Approval of the final version of the manuscript. CFAM has contributed en 1,2,3,4,5,6
Received: January 15, 2020; Accepted: November 24, 2020










 texto em
texto em