Serviços Personalizados
Journal
Artigo
Links relacionados
Compartilhar
Odontoestomatología
versão impressa ISSN 0797-0374versão On-line ISSN 1688-9339
Odontoestomatología vol.22 no.36 Montevideo 2020 Epub 01-Dez-2020
https://doi.org/10.22592/ode2020n36a11
Case Report
Follow up protocol in proliferative multifocal leukoplakia to avoid malignant transformation. A case report
1Facultad de Odontología, Universidad Nacional de Córdoba, Córdoba, Argentina
2Facultad de Odontología, Universidad Nacional de Córdoba, Córdoba, Argentina
3Cátedra de Estomatología, Facultad de Odontología, Universidad Nacional de Córdoba, Córdoba, Argentina. ggilligan@unc.edu.ar
4Cátedra de Estomatología, Facultad de Odontología, Universidad Nacional de Córdoba, Córdoba, Argentina
Keywords: leukoplakia; oral squamous cell carcinoma; follow-up; proliferative leukoplakia
Palabras Claves: leucoplasia; carcinoma de células escamosas; seguimiento; leucoplasia proliferativa
Palabras chaves: leucoplasia; carcinoma de células escamosas; seguimento; leucoplasia proliferativa
Introduction and background
Many potentially malignant disorders (PMDs) present in the oral mucosa usually manifest clinically as white lesions1. Among them, leukoplakia, and a very aggressive variant called proliferative verrucous leukoplakia (PVL), or better called proliferative multifocal leukoplakia (PML)2, has the highest malignancy rate. PML was described by Hansen et al.3 in 1985; its diagnostic criteria have been optimized by many authors to date4-5. The World Health Organization (WHO) defined PML as an aggressive variety of leukoplakia characterized by a high recurrence and malignancy transformation rate6.
PML initially presents as a white lesion and may correspond to a keratotic spot or plaque, which gradually becomes proliferative, covering different oral mucosa areas, becoming multifocal, with diffuse and uncontrollable growth. The lesions can become bulkier and resemble warts and become verrucous carcinomas or squamous cell carcinomas7.
PML lesions tend to resist treatment, including modalities such as laser excision, conventional surgery, radiation, and chemotherapy. However, strict patient follow-up, monitoring, and early biopsies are the best tool to prevent carcinomatous transformation5. This paper aims to report a clinical case of a patient with PML lesions and the strict clinical follow-up over three years (current status).
Description
In March 2017, a 75-year-old female patient was referred by a head and neck surgeon to Stomatology Department “A” of the School of Dentistry of Universidad Nacional de Córdoba, Argentina. She had white lesions on the edge of the tongue, without associated symptoms.
The woman, a total edentulous patient, had a health history of osteoarthritis and hypertension, with no other relevant personal, family, or genetic history. Associated habits such as tobacco, alcohol, and drug use were not reported. Her diet was rich in vegetables and fruits, and she had one liter of mate daily, whose temperature did not exceed 75°.
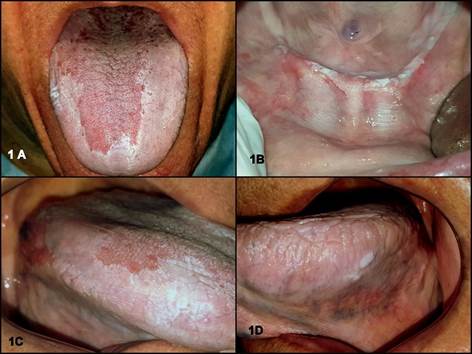
The intraoral exam showed an extensive white lesion of five years of evolution; it did not detach with the scraping and was located in the anterior sector of the inferior alveolar ridge, dorsal and ventral face, edges and tip of tongue, compromising the buccal mucosa equally on both sides. The lesions appeared by sectors as true keratotic plaques, which tend to become verrucous, as shown in Fig. 1.
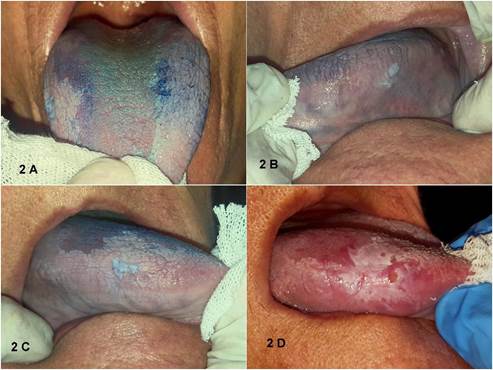
Presurgical laboratory tests were run, and we decided to perform serial incisional biopsies of the most representative areas stained with toluidine blue (Figure 2). This pigment does not easily penetrate the epithelium’s deep layers, so its use would be limited to verrucous or hyperkeratotic lesions. However, it remains a useful complementary tool to select the representative area before the surgical procedure. In addition, cells were collected by smear or brush. They were then deposited in a specific transport medium, the Polymerase Chain Reaction (PCR) was run to test for Human Papillomavirus (HPV) and its subsequent genotyping.
The anatomic pathology of the biopsied specimens showed similar images: a hyperplastic, acanthosic epithelium with superficial hyperkeratosis, hypergranulosis, and some cellular alterations affecting the basal layer, compatible with mild dysplasia. Clinical-pathological manifestations can correspond to the diagnostic criteria for proliferative multifocal leukoplakia. The PCR-HPV result was negative; therefore the presence of viral DNA in the lesions was ruled out.
As the lesions were multifocal and extensive, a follow-up protocol was implemented. This protocol-agreed with the patient and her relatives-established monthly appointments in the first semester, bimonthly in the second semester, and quarterly in the second year of follow-up. After that, and according to the evolution of the clinical picture, it was agreed to space appointments or reduce the time between appointments. Initially, a local antifungal treatment was provided, any traumatizing elements from the patient’s complete dentures were removed, and the patient was instructed on hygiene techniques. The importance of periodic check-ups was emphasized.
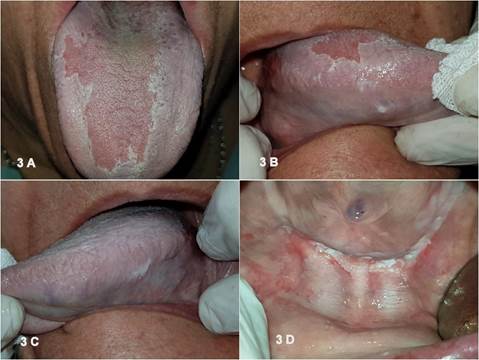
Subsequently, oral medicine residents were calibrated to standardize the follow-up criteria and follow a strict working methodology at the follow-up appointments. A general dental exam was performed, photographs of the affected and healthy oral mucosa were taken, and the area was stained with toluidine blue. A biopsy specimen was taken if there were new verrucous areas, nodular lesions with increased consistency, areas of rapid growth compared to the previous appointment, and erythroplasia that did not improve after trauma elimination or fungal infection treatment, or which was unrelated to these conditions). Fig.3
The patient remained stable for three years. A new biopsy specimen was taken due to the appearance of the above indication criteria, with a histopathological diagnosis of hyperkeratosis with mild dysplasia, similar to the initial lesions.
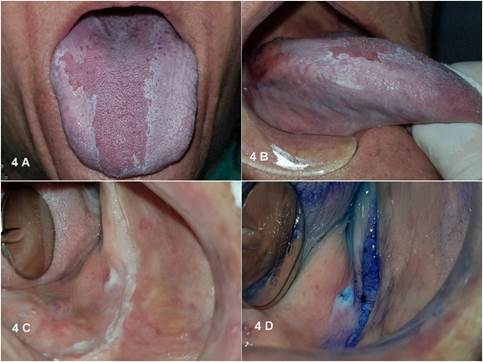
The patient attended the follow-up appointments in the first year; however, she did not in the following years due to mobility issues associated with her osteoarthritis. Until the time this paper was written, no focal points of carcinomatous transformation were observed. Fig. 4shows the latest follow-up visit and Fig. 5 shows the histopatology of the first tongue edge biopsy.
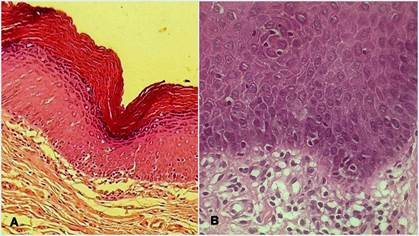
It must be noted that only three incisional biopsies were necessary throughout the follow-up: two at the start of the treatment and a third one when a suspicious lesion appeared. All the histopathological analyses showed hyperkeratosis and mild epithelial changes. A: Acanthosic epithelium with hypergranulosis and hyper-orthokeratinization. Low focal inflammatory activity was observed in the connective tissue. 3 B: some hyperchromatic nuclei and cytological and architectural signs of mild epithelial dysplasia are observed.
Discussion
PML is one of the pathologies that has promoted the most debate on its nomenclature, nature, and treatment. Over the past decades, different diagnostic criteria have been proposed (Ghazali et al., Gandolfo et al., Cerero-Lapiedra et al., Villa et al.). In many cases, it may begin as a single homogeneous leukoplakia8 that proliferates into a multicenter entity, with or without malignant changes at diagnosis. One of the most commonly used diagnostic criteria are those proposed by Cerero-Lapiedra et al. in 20114. Our patient was a 70-year-old woman with no associated risk factors such as tobacco and alcohol. Clinically it was a white plaque measuring over 3 cm, with verrucous areas that involved more than two different sites in the oral cavity: dorsal and ventral face, edges and tip of the tongue, buccal mucosa, and lower alveolar ridge, showing that the lesions spread throughout the evolution. All these characteristics coincide with similar cases of PML reported in the literature over the years and met all the major and minor criteria of proliferative multifocal leukoplakia proposed by Cerero-Lapiedra et al. in 2011.
It is recommended that photographs be taken at each visit as part of the clinical management and follow-up to record lesions and areas of clinically healthy mucosa. Biopsies are indicated when there are clinical findings of red, nodular, or verrucous areas or induration areas. In cases of reported dysplasia, lesions should be surgically removed, and pathologists should always inform the PML margins, even in the absence of dysplasia5. This patient underwent a strict follow-up during the first years, with detailed clinical monitoring of the lesions and ongoing check-up of the prosthesis condition to avoid chronic mechanical irritation and fungal superinfection.
The pathogenesis of the disease has not yet been established9. Given the verrucous clinical appearance of PML-associated lesions, HPV infection was possible; various HPV infection rates in PML are found in the literature10. In addition, aberrations have been found in cell cycle regulatory genes, with deletions, loss of heterozygosity, and mutations in some of these lesions11. In our case, PCR for molecular diagnosis of HPV was negative and the pathology could not be linked to patient habits or other external factors (such as living in an area with high consumption of water contaminated with arsenic, frequent in our country), associated with the presence of oral leukoplakia.
Some authors have proposed that some PML could initially have clinical forms similar to those reported in oral lichen planus (OLP) and lichenoid lesions12. The following might link PML and OLP: PML frequency in female patients, non-smokers, multifocal lesions, usually over the age of 50, and the similarity of this clinical scenario with OLP13. In this case, the patient’s characteristics coincide with the features described above. In addition, there are clinical details that led clinicians to believe that this PML was associated with a previous OLP. The lingual dorsal lesion showed a true keratotic zone with a striated pattern, more so towards the tip, similar to those described in OLP. However, as there were no oral mucosal biopsies before the patient’s first appointment, OLP could not be initially diagnosed. Some authors have also proposed similar clinical findings, where PML lesions can coexist with lichenoid areas5,14-15. In some reported cases of PML, a juxtaepithelial interface lymphocytic infiltrate similar to that of OLP was observed16-17, sparking some controversy on the true nature of PML. The juxtaepithelial infiltrate present in many white lesions would not be exclusive of pathologies such as OLP or lichenoids, as it is a non-specific histological finding present in dysplastic, allergic lesions associated with mechanical irritation14.
PML is not only characterized by its high rate of post-surgical recurrence, but by its high rate of carcinomatous transformation: it is the PMD with the most significant potential to become a carcinoma. Transformation rates range from 60 to 100%, with the gingiva and palate being the two areas with the highest frequency of transformation8-9,16,18-19. In our case, the lesions appeared in the edentulous ridge, tongue, buccal, and lip mucosa, and there were no palatal lesions. We also observed how the mucosa quickly healed with a keratinizing pattern once the lesions in the incisional biopsies were surgically removed. Strict follow-up protocols and the patient’s adherence to them allowed for a careful examination of the lesion’s evolution by performing early biopsies in the event of significant changes. As it is a refractory pathology, it is essential to monitor the patient over time.
Finally, follow-up procedures for patients with PML were systematized, including this case. Controls included: a systematic exam of the entire oral mucosa, both visual and through palpation, functional static and dynamic control of prosthesis, periodontal health (not necessary since the patient was edentulous), and possible fungal superinfection. The presence of new lesions or increased lesions compared to the previous visit is evaluated. In these patients, medical records and photographic material of pathological and healthy mucosa are carefully studied to establish if the pathology is progressing or if there are moments of remission of the proliferative pattern. Once all these aspects have been evaluated, lesions and healthy mucosa are stained with toluidine blue, as these patients are considered at high risk of developing carcinomas, specifically on the edges of the tongue and floor of the mouth. Then the staining results are observed and complemented with lesion palpation. Based on the analysis of all these variables during follow-up, incisional biopsies are performed if any suspicious lesions are found.
Conclusion
PML is an oral mucosa disease that has the highest rate of carcinomatous transformation. Successful treatment of these patients entails avoiding such transformation. In cases as widespread as the one presented here, where total removal of the lesion is impossible, a comprehensive follow-up is the most critical preventive-therapeutic measure.
In these cases, efforts should aim to create the necessary conditions for patients and their environment to foster empathy with the oral medicine service where they are being treated to achieve adherence to the follow-up plan. Early biopsies, when there are clinical alert changes, are the first step in preventing PML carcinomatous transformation.
REFERENCES
1. Warnakulasuriya S. Clinical features and presentation of oral potentially malignant disorders. Oral Surg Oral Med Oral Pathol Oral Radiol. 2018; 125 (6): 582-90. [ Links ]
2. Aguirre-Urizar JM. Proliferative multifocal leukoplakia better name that proliferative verrucous leukoplakia. World J Surg Oncol. 2011; 10(9): 122. [ Links ]
3. Hansen LS, Olson JA, Silverman S. Proliferative verrucous leukoplakia. A long-term study of thirty patients. Oral Surg Oral Med Oral Pathol. 1985; 60 (3): 285-98. [ Links ]
4. Cerero-Lapiedra R, Baladé-Martínez D, Moreno-López L-A, Esparza-Gómez G, Bagán JV. Proliferative verrucous leukoplakia: a proposal for diagnostic criteria. Med Oral Patol Oral Cir Bucal. 2010;15 (6): e839-845. [ Links ]
5. Villa A, Menon RS, Kerr AR, De Abreu Alves F, Guollo A, Ojed D, Woo SB. Proliferative leukoplakia: Proposed new clinical diagnostic criteria. Oral Dis. 2018; 24 (5): 749-60. [ Links ]
6. El-Naggar AK, Chan JKC, Grandis JR, Takata T, Slootweg PJ.. WHO Classification of Head and Neck Tumours (Internet). (cited 2020 Jan 27). Available from: Available from: https://publications.iarc.fr/Book-And-Report-Series/Who-Iarc-Classification-Of-Tumours/Who-Classification-Of-Head-And-Neck-Tumours- 2017 [ Links ]
7. Palefsky JM, Silverman S, Abdel-Salaam M, Daniels TE, Greenspan JS. Association between proliferative verrucous leukoplakia and infection with human papillomavirus type 16. J Oral Pathol Med. 1995; 24 (5): 193-7. [ Links ]
8. van der Waal I, Reichart PA. Oral proliferative verrucous leukoplakia revisited. Oral Oncol. 2008; 44 (8): 719-21. [ Links ]
9. Capella DL, Gonçalves JM, Abrantes AAA, Grando LJ, Daniel FI. Proliferative verrucous leukoplakia: diagnosis, management and current advances. Braz J Otorhinolaryngol. 2017; 83 (5): 585-93. [ Links ]
10. Campisi G, Giovannelli L, Ammatuna P, Capra G, Colella G, Di Liberto C, et al. Proliferative verrucous vs conventional leukoplakia: no significantly increased risk of HPV infection. Oral Oncol. 2004;40 (8): 835-40. [ Links ]
11. Kresty LA, Mallery SR, Knobloch TJ, Li J, Lloyd M, Casto BC, et al. Frequent alterations of p16INK4a and p14ARF in oral proliferative verrucous leukoplakia. Cancer Epidemiol Biomarkers Prev. 2008; 17 (11): 3179-87. [ Links ]
12. Garcia-Pola M-J, Llorente-Pendás S, González-Garcia M, García-Martín J-M. The development of proliferative verrucous leukoplakia in oral lichen planus. A preliminary study. Med Oral Patol Oral Cir Bucal. 2016; 21 (3): e328-334. [ Links ]
13. Lopes MA, Feio P, Santos-Silva AR, Vargas PA. Proliferative verrucous leukoplakia may initially mimic lichenoid reactions. World J Clin Cases. 2015; 3 (10): 861-3. [ Links ]
14. Thomson PJ, Goodson ML, Smith DR. Potentially malignant disorders revisited-The lichenoid lesion/proliferative verrucous leukoplakia conundrum. J Oral Pathol Med. 2018; 47 (6): 557-65. [ Links ]
15. Fitzpatrick SG, Honda KS, Sattar A, Hirsch SA. Histologic lichenoid features in oral dysplasia and squamous cell carcinoma. Oral Surgery, Oral Medicine, Oral Pathology and Oral Radiology. 2014; 117 (4): 511-20. [ Links ]
16. Cabay RJ, Morton TH, Epstein JB. Proliferative verrucous leukoplakia and its progression to oral carcinoma: a review of the literature. J Oral Pathol Med. 2007; 36 (5): 255-61. [ Links ]
17. Morton T, Cabay R, Epstein J. Proliferative verrucous leukoplakia and its progression to oral carcinoma: Report of three cases. J Oral Path Med. 2007;36. [ Links ]
18. Munde A, Karle R. Proliferative verrucous leukoplakia: An update. J Cancer Res Ther. 2016; 12 (2): 469-73. [ Links ]
19. Bagan JV, Jimenez Y, Sanchis JM, Poveda R, Milian MA, Murillo J, et al. Proliferative verrucous leukoplakia: high incidence of gingival squamous cell carcinoma. J Oral Pathol Med. 2003; 32 (7): 379-82. [ Links ]
Authors' contribution note: 1.Conception and design of study 2.Acquisition of data 3.Data analysis 4.Discussion of results 5.Drafting of the manuscript 6.Approval of the final version of the manuscript. FL has contributed in 1, 2, 3 y 5. TM has contributed in 1, 2, 3 y 5. GG has contributed in 1, 5 y 6. PR has contributed in 5 y 6.
Received: April 09, 2020; Accepted: July 10, 2020










 texto em
texto em