Servicios Personalizados
Revista
Articulo
Links relacionados
Compartir
Odontoestomatología
versión impresa ISSN 0797-0374versión On-line ISSN 1688-9339
Odontoestomatología vol.22 no.36 Montevideo 2020 Epub 01-Dic-2020
https://doi.org/10.22592/ode2020n36a9
Update
Bone regeneration as an example of tissue engineering in dentistry, with an emphasis on the development of scaffolds
1 Facultad de Odontología, Universidad de la Republica, Montevideo, Uruguay
2 Facultad de Odontología, Universidad de la Republica, Montevideo. Uruguay
3 Área de Patología Molecular Estomatológica, Facultad de Odontología, Universidad de la Republica, Montevideo. Uruguay
4 Cátedra de Histología, Facultad de Odontología, Universidad de la Republica. Montevideo. Uruguay
Tissue engineering is a multidisciplinary scientific area that has the therapeutic purpose of restoring, replacing, or increasing the functional activities of organic tissues.
Objective:
This work aims to review the literature on tissue engineering in oral and maxillofacial procedures.
Methods:
A bibliographic search was conducted in PubMed MEDLINE, Google Scholar, and LILACS, using the terms “stem cells,” “bone regeneration,” and “tissue growth factors.”
Discussion:
Several biomaterials capable of promoting bone neoformation have been described. They need to be adequately manipulated and have the right architecture and achieve the synergy of the various properties.
Conclusions:
Scaffolds have the widest uses, and selecting one does not depend on the material itself.
Keywords: tissue engineering; stem cells; bone regeneration; growth factors
La ingeniería tisular es un área científica multidisciplinaria la cual tiene como finalidad terapéutica restaurar, sustituir o incrementar las actividades funcionales de los tejidos orgánicos.
Objetivos:
Realizar una revisión de la literatura sobre la ingeniería tisular a nivel del área bucomaxilofacial.
Métodos:
Se realizó una búsqueda bibliográfica mediante los portales PubMed MEDLINE, Google Scholar, y LILACS, usando los términos “células madre, regeneración ósea y factores de crecimiento tisular”.
Resultados:
Se obtuvieron 193 resultados positivos, de los cuales 24 se utilizaron para el desarrollo del presente trabajo.
Discusión:
Han sido expuestos varios biomateriales capaces de propiciar la neoformación ósea, siendo esencial su correcta manipulación, la conformación de una arquitectura adecuada y la sinergia de las diversas propiedades.
Conclusiones:
Los andamios son los que brindan mayor oferta para su uso y la elección de cada uno de ellos no depende del material en sí mismo.
Palabras clave: ingeniería tisular; células madre; regeneración ósea y factores de crecimiento
A engenharia de tecidos é uma área científica multidisciplinar cujo objetivo é restaurar, substituir e aumentar as atividades funcionais dos tecidos orgânicos.
Objetivos:
O objetivo deste trabalho é revisar a literatura sobre engenharia de tecidos no nível da área bucomaxilofacial.
Métodos:
Foi realizada uma pesquisa bibliográfica no portals PubMed MEDLINE, Google Scholar, e LILACS, utilizando os termos “células-tronco, regeneração óssea e fatores de crescimento tecidual”.
Resultados:
foram obtidos 193 resultados positivos, dos quais 24 foram utilizados para o desenvolvimento deste trabalho.
Discussão:
Vários biomateriais capazes de promover a neoformação óssea foram expostos, sendo sua manipulação correta, a conformação de uma arquitetura adequada e a sinergia das várias propriedades.
Conclusões:
são os andaimes que oferecem a melhor oferta para seu uso e a escolha de cada um não depende do material propriamente dito.
Palavras chaves: engenharia de tecidos; células-tronco; regeneração óssea e fatores de crescimento
Introduction
Tissue engineering is an ever-expanding multidisciplinary scientific area that aims to create a tissue equal or as close as possible to the damaged tissue. In recent years, it has become increasingly interesting within the health sciences, which has led to many research studies. Its therapeutic use aims to restore, replace, or increase organic tissues’ functional activities1-2. It is based on knowledge of histology, a discipline-from the field of teaching-conditioned by research. Its ultimate goal is to create new tissue3-4.
This requires isolating and culturing cells in the laboratory and having biomaterials that can replace extracellular matrices, which should have similar behavior to the tissues they replace3.
This paper aims to conduct a literature review of the tissue engineering journey applied to bone tissue in oral and maxillofacial work.
Methodology
A literature search was conducted in May 2019, using the PubMed MEDLINE database, Google Scholar, and LILACS, including papers from the last six years. The following descriptors were used: "stem cells," "bone regeneration," and "growth factors." Search strategy: (“Stem Cells”[Mesh]) AND “Bone Regeneration”[Mesh] AND “growth factors” AND (“last 6 years ). Articles were included in English and Spanish and based on case reports, literature reviews, and systematic reviews. In total, 193 positive results were obtained, of which 24 were used for this study.
Development
Origin and evolution of cell theory
In the early 17th century, cells were first seen and described by A. van Leeuwenhoek (1632-1723), a Dutch naturalist who devoted his work to researching cells with magnifying glasses5.
Georges Buffon, a French naturalist (1707-1788), stated that microscopic beings represent living molecules that are joined together and form animals. Together with experience and microscopic observation, all these philosophical ideas led to the theory that small “pores” appear in animal and plant bodies, now known as cells5.
Wolff was a professor of philosophy and the founder of the epigenetic theory of evolution, which proposed that during the development of the individual, new structures are formed from undifferentiated material, with the help of an essential force5.
However, it was the English botanist Robert Hooke who, in 1665, introduced the word cell to designate the first chambers he had observed when studying thin sheets of plant tissues under the microscope. While he was a pioneer in terminology and describing its structure and skeleton, he never grasped its true meaning. It was not until the mid-19th century that the German scientists Schleiden and Schwann discovered the cellular nature of living matter by claiming that organisms are structures made up of other lower beings which, according to specific rules, combine to compose them. Both concluded that the cell is the basic structural unit of life. They argue in their cellular theory that all living things are composed of one or more cells5.
Rudolf Virchow (1821-1902), in his work “Cellular Pathology” (1858), considered the cell as the basic metabolic and structural unit of living beings, and proposed that all cells come from other (pre-existing) cells5.
What Schleiden and Schwann proposed in 1839, in addition to what Virchow stated in 1858, contributed extensively to cellular theory as it is known today, which can be summarized in the following principles:
1-The vital functions of organisms occur in cells or their immediate environment, controlled by substances they secrete. The cell includes all vital functions; one cell is enough to have a living thing. The cell is the physiological unit of life.
2-All cells arise from pre-existing cells through cell division. It is the unit of origin of all living things.
3. Each cell contains all the inherited information necessary to control its cycle and the development and functioning of an organism of its species and transmit it. The cell is the genetic unit5.
Evolution in stem-cell research
Ernst Haeckel (German biologist, physician, and philosopher) introduces the term Stammzelle (stem cells) to name the unicellular organism that was the common ancestor of all multicellular organisms. This ancestor would originate from the most primitive life forms he called Moneren (from the Greek moneres, “simple”). The German doctor studied and compared the development of embryos of various species, which led him to conclude that the earlier the stage observed, the more individuals of different species resemble each other, reaching the point of being indistinguishable. It was then that he formulated the “biogenetic law”, which states that each embryo’s developmental stage represents an accelerated and summarized form of an evolutionary ancestor6.
In this context, Haeckel used the term stem cell again to refer to the fertilized egg or zygote. As it is the earliest stage of development, it would represent the common origin of all multicellular organisms, which makes it special character clear as it is the precursor cell of all cells in the adult organism6.
In the course of the 1890s, August Weismann argues in his theory of the continuity of germ plasma that multicellular organisms consist of germ cells that contain and transmit heritable information and somatic cells, which carry out ordinary bodily functions. Within this context, Theodor Bovery (1862-1927) depicted embryonic stages of the Ascaris megalocephala worm and observed that stem cells, primordial germ cells, were a subgroup of zygote-derived cells that during development had the particularity of dividing, giving rise to both a somatic cell precursor cell and another self-similar cell6.
It must be noted that at this point in history, the essential characteristics that define the concept of stem or stem cells today had already been described: they are undifferentiated cells (undifferentiation) that can give rise to cells identical to themselves (self-renewal) and at the same time to differentiate themselves from other types of specialized cells (plasticity)6.
At around the same time, the hematopoietic system was studied: its formation, development, and regeneration. Based on the study of stem cells in embryology, the question arises whether there exists a common precursor to the different blood cell types. Artur Pappenheim (1870-1916) devoted himself to studying hematopoiesis that occurred during the embryonic development of amphibians. He made diagrams to represent the precursor blood cell giving rise to the various blood-cell bloodlines. In one of these works, he proposed the name Mutterzellen (stem cell) to name the common precursor, in Berlin in 1896. However, it was the Russian researcher Alexander Maximov (1874-1928) who, in 1909, officially used the term Stamzelle to name the precursor cell, stating its properties-undifferentiated, self-renewal, and plasticity-, which had already been described in embryology. In 1963, Canadian researchers Ernest McCulloch and James Till conducted experiments involving injecting bone marrow (BM) cells into irradiated mice. They observed that small raised lumps grew on their spleens in proportion to the number of BM cells injected6. Each lump originated from a single cell that they called a colony-forming unit (CFU). In collaboration with Lou Siminovitch, these researchers found evidence that these undifferentiated cells were capable of self-renewal and of giving rise to all blood components, fulfilling the characteristics of a stem cell and proving the existence of the common blood precursor cell. Such CFU would subsequently be called a hematopoietic stem cell6.
In 1975, Dr. Rheinwald’s team established the standard method, which provided the necessary and fundamental conditions to grow this type of cells in culture indefinitely7.
In 1980, the first clinical application of stem cells was achieved by Dr. Banks-Schelegel’s team. They obtained skin epithelium in vitro and demonstrated its viability by using it as a graft on experimental animals.
In 1981, British scientists Martin Evans and Matthew Kaufman successfully isolated and cultured mouse embryonic stem cells from the internal cell mass of blastocysts8.
The term stem cell officially entered the scientific scene when histo-embryologists Theodor Boveri and Valentin Haeckel used it. They described the hereditary characteristics of germ cells and their pluripotency, as well as their self-renewal8.
In 1998, Science magazine published that Professor Thomson from the University of Wisconsin had developed the first line of human embryonic stem cells, successfully derived from the internal cell mass of a blastocyst produced by in vitro fertilization8.
Tissue engineering today
The term tissue engineering was introduced in 1987 by Fung. It is an interdisciplinary scientific field that develops artificial biological tissues and their therapeutic use to restore, replace, or increase the functional activities of organic tissues. It is based on the use of living cells, manipulating the extracellular environment, and creating biological substitutes that will eventually be implanted in the body. It is an ever-expanding area, which is based on knowledge of histology. Its ultimate aim is to develop new tissue from cells derived from cultures and biomaterials that will serve as support9.
The research and development process in the area has contributed significantly to establishing tissue engineering as a viable treatment option in medicine and dentistry.
Three components are essential in this field: stem cells, scaffolds, which are insoluble substrates, and growth factors, which produce the osteoinductive signal for bone neoformation7. As the three of them are equally necessary for cell therapy, we describe each of them below.
Stem cells are capable of dividing continuously and producing progenitor cells that can give rise to specialized cells. Their remarkable ability to differentiate themselves is called plasticity10.
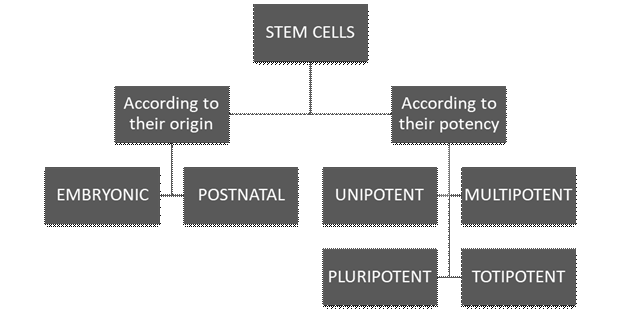
We can classify stem cells according to their origin and their potential to form specialized cells. They can also be divided according to their potential; cell potency is a cell’s ability to differentiate into other cell types.
Regarding their origin, there are embryonic stem cells and postnatal stem cells. Embryonic cells are produced from egg fertilization. Once this occurs, the blastocyst is formed: a sphere of about 100 cells, with an outer layer called the trophoblast, a fluid-filled cavity, the blastocele, and a cell mass inside. It would be possible to isolate stem cells in the blastocyst stage11.
On the other hand, we have postnatal stem cells: undifferentiated cells located in tissues or organs, among other already specialized cells. They can differentiate according to the needs of the tissue or organ where they reside, contributing to the organism’s homeostasis and tissue repair11.
They can also be divided according to their potency: cell potency is a cell’s ability to differentiate into other cell types. In this category, we find totipotent, pluripotent, multipotent, and unipotent stem cells. Totipotent cells can divide and produce all adult cell types and can even give rise to an entire individual. This is a quality of embryonic stem cells. Pluripotent stem cells can give rise to differentiated cell types derived from endodermal, mesodermal, or ectodermal tissue. Multipotent cells are differentiated cells that can form various tissue types from fetal tissue, umbilical cord blood, or postnatal stem cells. Finally, unipotent cells can form only one particular cell type11 (See Figure 1)10)
Table 1: Postnatal stem cells differentiated into mesenchymal and epithelial cells
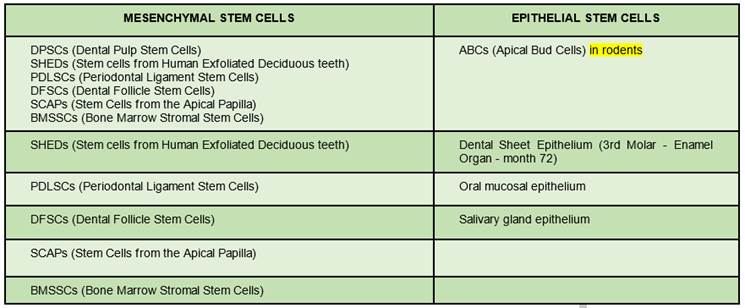
(Prepared by the authors.)
Table 1: shows postnatal dental stem cells. Another basic element in tissue engineering are scaffolds, which act as a temporary extracellular matrix12. They create the necessary three-dimensional microenvironment to achieve cell growth and differentiation, and promote cell attachment and migration, thus facilitating the development of functional tissues and organs10.
Scaffolding must meet several requirements to achieve such conditions: high porosity and adequate pore size, necessary to facilitate nutrient seeding and diffusion through cell structure; large surface area and good degradation. They must be biocompatible and interact positively with other attachment, growth, and migration cells. In addition, they must have good physical and mechanical resistance10.
Scaffold design in dentistry is complex since it must fit into a three-dimensional anatomical defect and must be temporarily released from loads until new bone tissue is formed12-13.
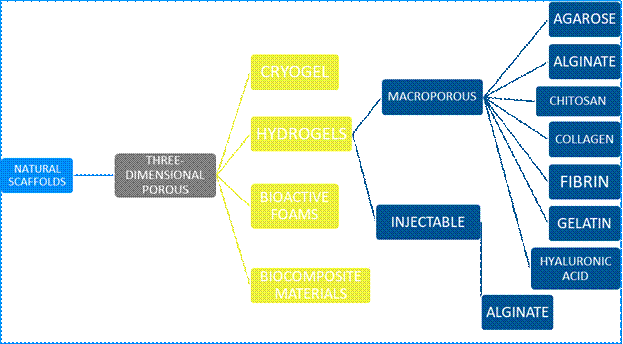
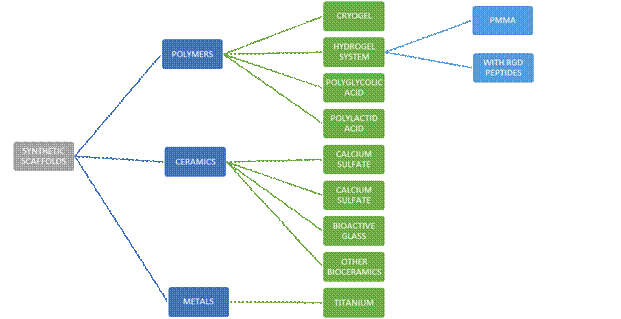
Depending on their composition, scaffolds can be classified as:
natural, organic, or biodegradable
synthetic, inorganic, or permanent12.
The first type is developed with extracellular matrix components, protein derivatives such as collagen, fibrinogen, hyaluronic acid, glycosaminoglycans, hydroxyapatite, etc.12.
They have the advantage of being bioactive, biocompatible, and similar to natural tissue regarding their mechanical properties. Their disadvantages are that they allow limited control over physicochemical properties, they have a low degradation rate, and pathogen sterilization and purification when isolated from different sources12.
Within this group, we find three-dimensional porous scaffolds (Figure 2). They are highly porous interconnected pore networks with a diameter of at least 100µm to facilitate cell growth, vascularization, temporary extracellular matrix production, and waste removal12.
Hydrogel-based scaffolds are among the most commonly used scaffolds; they can be macroporous or injectable. They are composed of hydrophilic polymers that form three-dimensional networks. They usually swell readily in contact with water, form macromolecules that are structurally similar to body components, and have excellent biocompatibility with a minimal inflammatory response. Their hydrated structure allows for the transport of soluble factors, nutrients, and waste. Pore size and distribution, and interconnectivity are important factors governing the use of hydrogels in tissue engineering. This is critical for cell distribution in the gel12-13.
Alginate, a natural polymer, is a well-established biomaterial due to its low cost, biocompatibility, and mild gelation. It is widely used in cartilage and bone tissue repair. However, its biocompatibility depends on many factors such as its composition, molecular weight, and contaminants in the gel12-13.
Injectable hydrogels have gained considerable attention because they form an in vivo gel. This is an excellent advantage for tissue engineering, as in situ gelation of the liquid to hydrogel materials is possible. This allows hydrogels to fill defects of any size or shape12-13.
These materials also function as a dynamic liquid support system for transporting living cells, medications, and growth factors required for tissue regeneration12-13.
Alginate microspheres loaded into a calcium phosphate paste were tested for injectability in order to reconstruct craniofacial bone tissue. The results showed improved osteogenic differentiation with calcium, phosphate, and chitosan12-13.
Collagen has received increasing attention due to its excellent biocompatibility, ability to degrade into physiological products, and desirable interaction with cells and other macromolecules. The main drawback of pure collagen scaffolding lies in its low mechanical properties, which are not close to those of natural bone tissue14-16.
Synthetic, inorganic, or permanent scaffolds include polymers, ceramics, and metals (Figure 3).
Cryogels are hydrogels synthesized at temperatures below zero to obtain good porosity and mechanical resistance. They are synthesized at low temperatures by chemical or physical products, through gelation of polymer chains, or free radical polymerization of monomer precursors.
Cryogels have negligible resistance to mass transfer due to microporosity and pore connectivity, allowing for gentle circulation of nutrients to growing cells12-13.
Polymethylmethacrylate (PMMA) injectable hydrogels have long been used to secure orthopedic implants in the human skeleton. PMMA is generally used as monomers with an initiator mixture, mixed at room temperature, which polymerizes when injected into the body. These polymers become gelatinous quickly with photoinitiators or thermal initiators. They are biodegradable, mechanically robust, and can support osteoblast growth. However, their uses are limited to the delivery of cells such as osteoprogenitor cells, because their crosslinking during photopolymerization may affect cell viability10.
Polyglycolic acid (PGA) and polylactic acid (PLA) are polymers frequently used in the development of 3-D synthetic scaffolds. These materials are hydrolytically degradable. The degradation rate can be easily controlled and, also, they are versatile for creating three-dimensional microenvironments. Its low bioactivity appears as its main disadvantage10.
Within ceramic materials, calcium phosphate (CaP) has been thoroughly researched in bone regeneration in the form of cements, coatings, and three-dimensional cellular scaffolding because it has excellent osteoconductive properties10,14.
It is biocompatible, low-cost, and easy to synthesize12-13. Its main advantages include its composition similarity to hydroxyapatite, the ability of osteoprogenitor cells to process and reabsorb CaP-based materials, and the complex but highly effective intracellular signaling system of osteogenesis that is triggered by the presence of soluble calcium and inorganic phosphates resulting from byproducts of CaP crystal dissolution.
Currently, a large number of CaP-based materials are marketed for bone regeneration14,16-18.
They are formed by combining concentrations of hydroxyapatite as the stable phase and calcium phosphate as the soluble phase. The bioactivity of these ceramics can be controlled by manipulating the proportions of those components12-13.
Calcium sulfate (CaSO4) has been used in implant clinical dentistry, craniofacial surgery, alveolar cleft correction in children, periradicular endodontic surgery, and orthopedic surgery, achieving effective and consistent results.
Calcium sulfate and calcium phosphate compounds mimic the mineral phase of bone tissue. These materials induce a biological response similar to that of bone remodeling, creating a calcium-rich environment in the implantation area.
Walsh et al.19 suggested that the decrease in pH and local acidity during calcium sulfate resorption caused demineralization of adjacent bone and release of matrix-bound BMPs. In addition, increased angiogenesis at sites treated with calcium sulfate could explain the good results reported11.
Some authors20 have recently claimed that Strontium folate-based scaffolds increase bone regeneration in vivo and propose them as a useful alternative for bone tissue regeneration in complicated defects20.
Furthermore, metals can be used to develop scaffolds. The most accepted one is titanium because it has great biocompatibility and high synergy with bone tissue. It is widely used in orthopedics and oral surgery10.
The osteoinductive and vasculogenic potential of scaffolding is enhanced with the right growth factors that induce chemotaxis, proliferation, and differentiation of encapsulated and surrounding cells14.
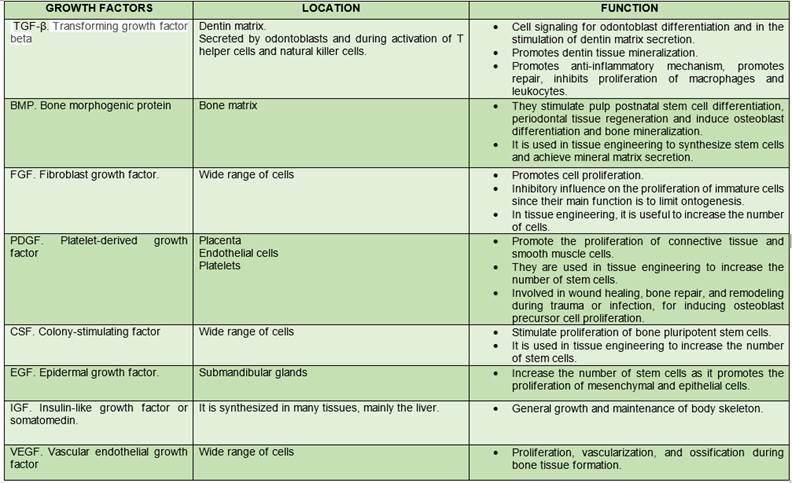
Growth factors are the third element necessary to trigger tissue engineering therapy. They are proteins that bind to receptors in the cell and induce cell proliferation and/or cytodifferentiation. They are used to control stem cell activity, increase proliferation rate, induce differentiation of cells into another tissue type, stimulate stem cells to synthesize and secrete mineralized matrix, and induce regeneration of injured tissues10.
Currently, we know two categories of biofactors that stimulate cell osteogenic activity. The first category induces non-osteoblast to osteoblast conversion. The second, increases osteoblast proliferation and activity, thus accelerating ossification and bone matrix formation, an effect known as modulation. Both induction and modulation work in synergy14,18,21-23) (Table 2).
Discussion
Tissue engineering proposes new innovative and revolutionary therapies that involve the regeneration or replacement of tissues or organs through three-dimensional structures that restore shape and function. This can be done from the patient’s own cells and biomaterials and biomolecules or from exogenous structures.
Dental regenerative techniques have advantages and disadvantages, and it is essential to know that some are currently under development14-15.
Thrivikraman et al. argue that biomaterials are used as biocompatible scaffolds that allow for migration, proliferation, and differentiation of both resident and external cells and are used to promote new bone tissue formation14-15. Mijiritsky et al. also propose that biomaterials are responsible for providing a three-dimensional substrate on which cells can proliferate, requiring essential growth factors for each cell type24.
Therefore, biomaterials are essential in bone regeneration since they promote and regulate a favorable microenvironment to trigger bone neoformation. In this work, we have described various biomaterials that can adapt to this role. It is essential to ensure biomaterials are correctly manipulated, as well as the formation of the necessary architecture, and the synergy of their various properties to promote the new tissue formation process12-13.
According to Shakya et al., designing a dental scaffold is not a simple task, as it must fit into a three-dimensional anatomical defect and be temporarily released from loads until the expected bone neoformation occurs12. The characteristics necessary to develop a dental scaffold must be considered: adequate porosity, biocompatibility, degradability, surface morphology, and mechanical resistance. Other characteristics such as bioactivity, promoting osteogenesis, and low cost also guide the selection of the right material. Within the range of biomaterials described, there is no one that meets all the ideal characteristics, so there is no ideal material to create a scaffold. Each case must indeed be studied to select the material that meets the most significant number of needs. Many times the best scaffold is developed by combining biomaterials. This aims to seek the synergy of its characteristics, while on other occasions, it is enough to choose one of them10,12-14.
Conclusions
We have made a historical journey on tissue engineering applied to the oral and maxillofacial field, especially bone tissue. We have analyzed its pillars: stem cells, growth factors, and scaffolds. Scaffolds exhibit the greatest number of uses, and selecting one does not depend on the material itself, since there is no ideal material, but rather on each particular case, as shown by our findings.
The application of bone regeneration treatments based on tissue engineering at the jaw level allows us to improve our patients’ quality of life through cutting-edge therapies.
REFERENCES
1. Piedra MAM, Espejo SA, Moral-Munoz JA,Campos F , Chato-Astrain J , Garcia-Garcia OD, Sanchez Porras D, Antonio Campos. An Evolutive and Scientometric Research on Tissue Engineering Reviews.Tissue Eng Part A. 2019; 26 (9-10) :569-577. doi: 10.1089/ten.TEA.2019.0247. [Epub ahead of print] [ Links ]
2. Espejo SA, Campos F, Piedra LM, Durand Herrera D, Moral-Munoz JA,Campos A, Piedra MAM. Global Tissue Engineering Trends: A Scientometric and Evolutive Study. Tissue Eng Part A. 2018; 24 (19-20): 1504-1517. [ Links ]
3. Piedra MAM, Alaminos M, Fernández Valadés Gámez R, España López A, Liceras-Liceras E, Sánchez Montesinos I, Martínez-Plaza A, Sánchez-Quevedo MC , R Fernández-Valadés, Garzón I. Development of a multilayered palate substitute in rabbits: a histochemical ex vivo and in vivo analysis. Histochem Cell Biol. 2017; 147(3):377-388. [ Links ]
4. Gómez de Ferraris, Campos Muñoz. Histología, embriología e ingeniería tisular bucodental, 2019, 4ta edición, editorial Médica Panamericana. P3-36. [ Links ]
5. Berón MP. Historia de la teoría celular. Universidad Nacional de Mar del Plata. 2006. [ Links ]
6. Apablaza F. Antecedentes históricos y conceptos básicos en el estudio de las células madre: células troncales, o la madre de todas las células. Rev. Actuali. Clinic. Meds. 2017; 1(1): p6-16 [ Links ]
7. Fernández González A, Lizana Moreno A, de Pablos Ramos M, Ruiz García A, Espinosa Ibáñez O, Fernández Porcel N, Guerrero Calvo J, Arrabal M, López-Carmona F, Arias-Santiago S. Optimización del cultivo de queratinocitos humanos para el desarrollo de un modelo de piel artificial humana: alternativas celulares como capa alimentadora. Real Academia de Medicina y Cirugía de Andalucía Oriental; Universidad de Granada. Agosto, 2016. [ Links ]
8. Smalley M, Ashworth A. Stem cells and breast cancer: a field in transit. Nature Reviews Cancer. 2003. 3: p832-844. [ Links ]
9. Gómez de Ferraris Ma.E, Campos Muñóz A. Histología, embriología e ingeniería tisular bucodental. Tercera edición. 2009. Editorial médica Panamericana. p2-26 p381-392. [ Links ]
10. Redón J, Jiménes L P, Urrego P A. Células madre en odontología. Revista CES Odontología. 2011; 24 (1) [ Links ]
11. Aquino-Martínez R, Angelo A.P, Ventura Pujol F. Calcium-containing scaffolds induce bone regeneration by regulating mesenchymal stem cell differentiation and migration. Stem cell research & therapy. 2017; 8: 265 p. doi:10.1186/s13287-017-0713-08. [ Links ]
12. Shakya A K, Kandalam U. Three-dimensional macroporous materials for tissue engineering of craniofacial bone. Br J OralMaxillofacSurg.2017; 55 (9): 875-891. [ Links ]
13. Hajibandeh J, Suzuki T, Fan A, Shang P, Mao JJ. Three-dimensional printed multiphase scaffolds for regeneration of periodontium complex. Tissue engineering Part A. 2014; 20 (7-8): 1342-51. [ Links ]
14. Thrivikraman G, Athirasala A, Twohig Ch, Kumar Boda S. Biomaterials for Craniofacial Bone Regeneration. Dent Clin North Am D. 2017; 61 (4): 835-856. Habraken W, Habibovic P, Epple M, Bohner M. Calcium phosphates in biomedical applications: materials for the future. Materials Today. 2016; 19 (2): 69-87. [ Links ]
Habraken W, Habibovic P, Epple M, Bohner M. Calcium phosphates in biomedical applications: materials for the future. Materials Today. 2016; 19 (2): 69-87. [ Links ]
15. Stoppel WL, et al. Clinical applications of naturally derived biopolymer-based scaffolds for regenerative medicine. Ann Biomed Eng. 2015; 43 (3): 657-80. [ Links ]
16. Kang Y, Kim S, Fahrenholtz M, et al. Osteogenic and angiogenic potentials of monocultured and co-cultured human-bone-marrow-derived mesenchymal stem cells and human-umbilical-vein endothelial cells on three-dimensional porous beta-tricalcium phosphate scaffold. Acta biomaterialia. 2012; 9 (1): 4906-15. [ Links ]
17. Tollemar V, Collier ZJ, Mohammed MK, Lee MJ, Ameer GA 4, Russell R. Stem cells, growth factors and scaffolds in craniofacial regenerative medicine. Genes Dis. 2016; 3 (1):56-71. [ Links ]
18. Walsh WR, Morberg P, Yu Y, Yang JL, Haggard W, Sheath PC, Svehla M, Bruce WJM. Response of a calcium sulfate bone graft substitute in a?confined cancellous defect. Clin Orthop Relat Res. 2003; 406:228-36. [ Links ]
19. Martín-Del-Campo M, Sampedro JG, Flores-Cedillo ML, Rosales-Ibañez R, Rojo L. Bone Regeneration Induced by Strontium Folate Loaded Biohybrid Scaffolds. Molecules. 2019;24 (9):1660. doi:10.3390/molecules24091660 [ Links ]
20. Habraken W, Habibovic P, Epple M, Bohner M. Calcium phosphates in biomedical applications: materials for the future? Materials Today. 2016; 19 (2). [ Links ]
21. Zhang B, Han Z, Duan K, Mu Y, Weng J. Multilayered pore-closed PLGA microsphere delivering OGP and BMP-2 in sequential release patterns for the facilitation of BMSCs osteogenic differentiation. J Biomed Mater Res Part A. 2018; Vol106A. p95-105. DOI: 10.1002/jbm.a.36210 [ Links ]
22. Shen XF, Zhang YX, Gu Y, Xu Y, Liu Y, Li B. Sequential and sustained release of SDF-1 and BMP-2 from silk fibroin-nanohydroxyapatite scaffold for the enhancement of bone regeneration. Biomaterials. 2016; 106: 205-216. [ Links ]
23. Mijiritsky E, Ferroni L, Gardin Ch, Bressan E, Zanette G, Piattelli A, Zavan B. Porcine Bone Scaffolds Adsorb Growth Factors Secreted by MSCs and Improve Bone Tissue Repair. Materials (Basel, Switzerland). 2017; 10 (9): 1054. doi:10.3390/ma10091054 [ Links ]
Authorship contribution note: 1) Conception and design of study 2) Acquisition of data 3) Data analysis 4) Discussion of results 5) Drafting of the manuscript SC has contributed in: 1, 2, 3, 4, 5, 6. RM has contributed in: 1, 2, 3, 4, 5, 6. VP has contributed in: 4, 5, 6. GP has contributed in: 4, 5, 6.
Received: March 05, 2020; Accepted: September 01, 2020











 texto en
texto en