Serviços Personalizados
Journal
Artigo
Links relacionados
Compartilhar
Odontoestomatología
versão impressa ISSN 0797-0374versão On-line ISSN 1688-9339
Odontoestomatología vol.22 no.35 Montevideo 2020 Epub 01-Jun-2020
https://doi.org/10.22592/ode2020n35a7
Research
Immunohistochemical profile of odontogenic myxoma, with emphasis on microvascular density and tumor aggressiveness markers
1 Departamento de Atención a la Salud, Universidad Autónoma Metropolitana (UAM), México
2 Área de Patología Molecular Estomatológica, Facultad de Odontología, Universidad de la República, Uruguay, csr_90@hotmail.com
3 Área de Patología Molecular Estomatológica, Facultad de Odontología, Universidad de la República, Uruguay
4 Área de Patología Molecular Estomatológica, Facultad de Odontología, Universidad de la República, Uruguay
5 Área de Patología Molecular Estomatológica, Facultad de Odontología, Universidad de la República, Uruguay
6 Universidad Autónoma Metropolitana de México, unidad Xochimilco, México
7 Patología Molecular Estomatológica, Facultad de Odontología, Universidad de la República, Uruguay
In order to elucidate better the biological behavior of the odontogenic myxoma (OM), immunohistochemistry was performed in 31 samples, using markers related to mechanisms of tumor progression (adhesion, angiogenesis, apoptosis, inflammation and cell proliferation). Odontogenic epithelium was detected in four samples with CK19 and CD138, the latter had a low-expression in extracellular matrix (ECM) and a high-expression in tumor cells. The mean microvascular density (MVD) assessed with CD34 and VEGF-A, was 7.51 and 5.35 blood vessels respectively. A high-expression of Orosomucoid-1 and Mast Cell Tryptase was observed in tumor cells and ECM, while Calretinin was completely negative. The previously mentioned immunohistochemical profile, as well as the low expression of Ki-67, Bcl-2 and p53 and the relatively low MVD, suggests that the proliferative, anti-apoptotic and angiogenic activities do not represent the main growing mechanisms of OM, which could be associated to other events, such as immunomodulation and ECM degradation.
Keywords: odontogenic myxoma; immunohistochemistry; tumoral markers; angiogenesis.
Con el fin de tener una mayor comprensión sobre el comportamiento biológico del mixoma odontogénico (MO), se realizó inmunohistoquímica en 31 muestras, utilizando marcadores relacionados con mecanismos de progresión tumoral (adhesión, angiogénesis, apoptosis, inflamación y proliferación celular). Epitelio odontogénico fue detectado en cuatro muestras mediante CK19 y CD138, este último, mostró expresión baja en matriz extracelular (MEC) y alta en las células tumorales. La microdensidad vascular (MDV) media fue de 7.51 y 5.35 vasos marcados con CD34 y VEGF-A respectivamente. Una alta expresión de Orosomucoide-1 y Mast Cell Tryptase se observó células tumorales y en MEC. El MO mostró negatividad para Calretinina. Este perfil inmunohistoquímico, la baja expresión para Ki-67, Bcl-2 y p53, y la relativamente baja MDV, sugieren que la actividad proliferativa, anti-apoptótica o angiogénica no representan los principales mecanismos de crecimiento del MO, los cuales podrían estar asociados a eventos como inmunomodulación y degradación de la MEC.
Palabras clave: mixoma odontogénico; inmunohistoquímica; marcadores tumorales; angiogénesis
Para melhor compreensão do comportamento biológico do mixoma odontogênico (MO), imuno-histoquímica foi realizada em 31 amostras, utilizando marcadores relacionados aos mecanismos de progressão tumoral (adesão, angiogênese, apoptose, inflamação e proliferação celular). Epitélio odontogênico foi detectado em quatro amostras por CK19 e CD138, o último mostrou baixa expressão na matriz extracelular (MEC) e alta em células tumorais. A microdensidade vascular (MDV) média foi de 7.51 e 5.35 vasos marcados com CD34 e VEGF-A, respectivamente. Uma alta expressão de Orosomucoide-1 e Mast Cell Tryptase foi observada nas células tumorais e na MEC. O MO mostrou negatividade para Calretinina. O perfil imuno-histoquímico mencionado acima, a baixa expressão de Ki-67, Bcl-2 e p53 e a relativamente baixa MDV, sugerem que a atividade proliferativa, anti-apoptótica ou angiogênica não representam os principais mecanismos de crescimento do MO, os quais poderiam estar associados com eventos como imunomodulação e degradação da MEC.
Palavras-chave: mixoma odontogênico; imuno-histoquímica; marcadores tumorais; angiogênese
Introduction
Odontogenic tumors (OT) include a group of lesions that primarily affect the gnathic bones, ranging from hamartomas to benign neoplasms and malignant tumors 1-2. Odontogenic myxomas (OM) are benign mesenchymal OT (with or without odontogenic epithelium); however, they exhibit an aggressive behavior: significant growth potential as well as a high recurrence rate (25%) 3.
Globally, OM represent between 2.2 and 17% of OT and affect mainly the posterior mandible, and occasionally the maxilla 4. Rare cases of peripheral OM have been reported, and they are less aggressive than central OM 5. OM occurs more frequently between the second and fourth decades of life (mean age of 28.6 years), and its clinical presentation is a slow growing volume increase, which is usually asymptomatic 6, so it may be large sized when diagnosed. Radiographically, its appearance may be unilocular (small lesions), or more commonly multilocular (classically described as “tennis racket” pattern) 3.
Microscopically, OM typically have fusiform to stellate cells dispersed in an abundant myxoid matrix composed mainly of glycosaminoglycans, which may or not have islands of odontogenic epithelium; the presence of collagen fibers is variable 3-4,6.
To date, there are few papers describing the molecular components of OM, so this study aims to determine the immunohistochemical profile of various tumor markers and to analyze microvascular density (MVD) in order to discuss its possible implications for the biological behavior of OM.
Methodology
Sample selection
Thirty-one cases of mandibular OM obtained from the Molecular Pathology Area of the School of Dentistry, Universidad de la República (Uruguay) were included. The samples were obtained by incisional biopsy, fixed in 10% formaldehyde and then included in paraffin blocks. This study was approved by the Ethics Committee of the School of Dentistry of Universidad de la República, under protocol number 091900-000113-14. Informed consent was obtained from all the study participants when the biopsy was performed.
Immunohistochemistry
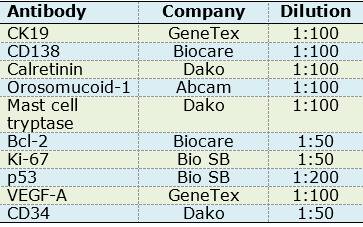
For immunohistochemistry assays, 3µm sections of paraffin embedded OM tissues were made and placed on slides; they were then deparaffinized in xylol and subsequently hydrated with decreasing concentrations of alcohol. Antigen retrieval was performed with a citrate solution (pH 6.2) in a pressure cooker in microwave oven on full power for one minute. Endogenous peroxidases were blocked with hydrogen peroxide at 0.9% for 5 minutes. Primary antibodies were incubated for one hour (Table 1).
Later the sections were incubated with the secondary biotinylated anti-mouse/anti-rabbit antibody and with the streptavidin/peroxidase complex (LSA-B + Dako Corporation, Carpinteria CA, USA) for 30 minutes each. The reaction was visualized with 3.3’-diaminobenzidine-H2O2 substrate (Dako Corporation, Carpinteria, CA, USA). Finally, the sections were contrasted with Mayer’s hematoxylin.
The following positive controls were used: oral mucosa for CK19 and CD138; breast cancer for calretinin; oral carcinoma for orosomucoid-1 and p53; intestine for mast cell tryptase, CD34 and VEGF-A; and tonsil for Bcl-2 and Ki-67.
Microscopic examination
Reactions were considered positive when brown labeling was observed in cells (tumor cells, endothelial cells or islands of odontogenic epithelium), or in extracellular matrix (ECM). Labeling patterns (nuclear, cytoplasmic, membranous, or ECM) varied for each type of antibody.
Proteins assessed in islands of odontogenic epithelium: CK19 and CD138
Using an optical microscope, each section was fully visualized under a 10x objective to identify areas with positive results and then at 40x to confirm it was odontogenic epithelium. The result was expressed as positive or negative.
Proteins assessed in tumor cells and ECM: CD138, calretinin, orosomucoid-1, mast cell tryptase, Bcl-2, Ki-67, p53 and VEGF-A
The complete sections were visualized under the 40x objective to determine the immunoexpression percentage throughout the tumor tissue on the slide. Percentages were classified into four groups: 0%, negative expression; 1% to 10%, low expression; 11% to 50%, moderate expression and greater than 50%, high expression 7.
Proteins assessed in blood vessels: VEGF-A and CD34
The method described by Weidner et al. was used to determine MVD. Tissues were initially visualized using a 10x objective to identify three areas with the highest concentration of positive vessels (hot spots), where vessel counting was performed manually using a 40x objective. Finally, the average number of vessels in the five fields of each sample was calculated 8.
Results
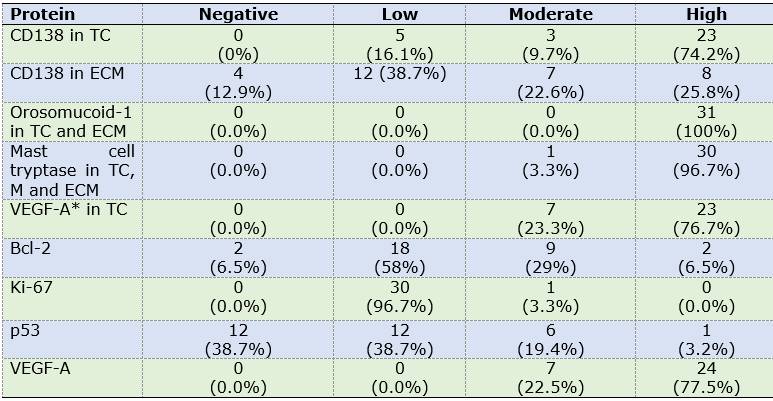
Odontogenic epithelium positive for CK19 and CD138 was detected in four cases. Additionally, CD138 showed high immunopositivity in tumor cells from most samples (74.2%) and low expression in ECM in 38.7% of cases (Figures 1A, B).
Calretinin was negative in all OM samples. In contrast, high immunopositivity of orosomucoid-1 was observed in tumor cells as well as ECM in 100% of cases, while mast cell tryptase was detected in the form of mast cell granules, focusing on ECM and with high expression in tumor cells: 96.7% of cases (n=30) (Figures 2 A, D) (Figures 2 C, E, F).
Low labelling for Bcl-2 (cytoplasmic), Ki-67 (nuclear) and p53 (nuclear) was observed in tumor cells (n=18, 58.1%; n=28, 90.3% and n=12, 38.7% respectively).
CD34 and VEGF-A were positive in endothelial cells (blood vessels). Additionally, VEGF-A showed a predominantly high expression in OM tumor cells (n=24, 77.5%) (Table 2). MVD was 7.51 and 5.35 for CD34 and VEGF-A respectively (Figures 1C and 2D).
Most proteins that were detected in tumor cells and ECM showed a high immunoexpression, except for Bcl-2. Table 3 shows the pattern, level and immunoexpression distribution of each protein in OM cases.
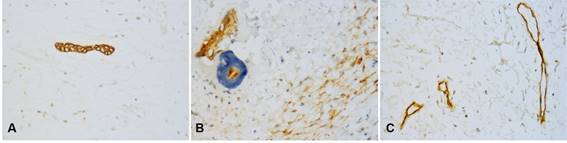
Fig. 1: Immunohistochemical markers in OM. Small islands of odontogenic epithelium were positive for CK19 (A) and CD138 (B), which was also expressed in tumor cells and ECM. Only endothelial cells in blood vessels were positive for CD34 (C). (Original magnification, A-C: 400x).
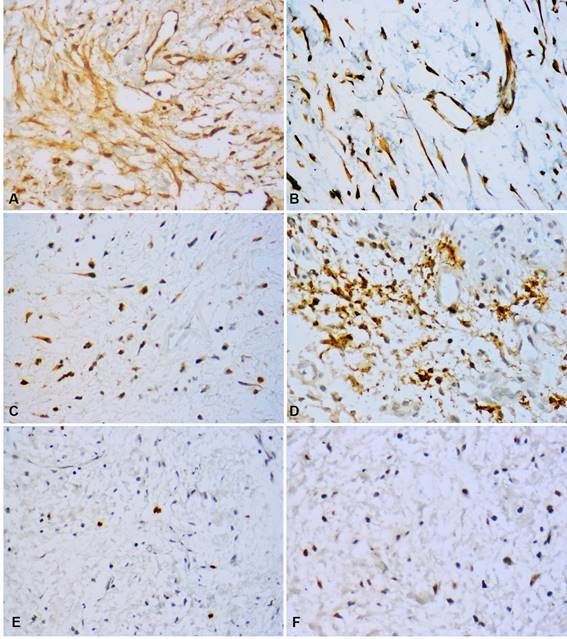
Fig. 2: Immunohistochemical profile of OM. High immunopositivity for orosomucoid-1 in tumor cells, endothelial cells and ECM (A). VEGF-A expression in tumor cells and endothelial cells (B). Bcl-2 expression in some tumor cells (C). Mast cell tryptase showed intense positivity with granular appearance in tumor cells, mast cells and ECM (D). Few nuclei positive for Ki-67 (E) and p53 (F). (Original magnification, A, B, C, E, F: 400x; D: 600x).
Table 2: Distribution of marker expression in OM components
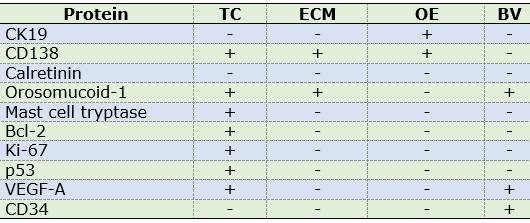
Abbreviations. TC: tumor cells, ECM: extracellular matrix, OE: odontogenic epithelium, BV: blood vessels.
Discussion
It has been suggested that OM has an odontogenic origin based on the occasional presence of small islands of odontogenic epithelium, its appearance in the mandible and maxilla, and its histomorphological similarity to the mesenchymal tissue of the tooth germ 9.
In this study, 13.3% of the sample had CD138 and CK19 positive odontogenic epithelial islands. This is consistent with two previous studies where epithelial islands were detected using CK19 in 4.8% and 14% of cases 9-10). Additionally, our results ratify the usefulness of CD138 as an alternative marker of odontogenic epithelium in OM.
A high expression of CD138 was observed in tumor cells in most cases (73.30%), while in ECM, immunoexpression was mainly low (46.70% of cases). Contrary to our results, in the study conducted by Etemad-Moghadam et al. (2017), CD138 was negative in all the samples 11. The physiological role of CD138, jointly with the ECM, is to participate in the induction and regulation of proliferation by interacting with families of heparin-binding growth factors 12. Additionally, it interacts with other ECM components 13, some of which (type I collagen, fibronectin and tenascin) are part of the ECM of the OM 14. This suggests that, in addition to the structural maintenance function of an epithelial adhesion protein, upon release into the ECM, CD138 could participate in various signaling pathways by interacting with growth factors and other molecules present in the ECM.
Although calretinin expression has been described in ameloblastomas, and has been associated with enamel production in tooth germ, in this study it was negative in OM, as in previous studies 15-16. This absence of calretinin can be explained by the mesenchymal origin of OM, unlike ameloblastomas and the enamel organ 17-18.
A previous study determined MVD in OM by CD34 expression, with similar results to ours 7. Ameloblastoma has higher MVD compared to other odontogenic tumors and cysts, suggesting an association with a more aggressive behavior 19-20. In our study, MVD in OM was five times lower than in the ameloblastomas of the study conducted by Seifi et al. (2001), suggesting that angiogenesis could partially contribute to tumor growth in OM 19.
In OM, VEGF-A (one of the majors signaling proteins for angiogenesis) was expressed in endothelial cells and tumor cells. Recent researches suggest that VEGF-A immunoexpression in the epithelium of odontogenic cysts and epithelial OT, such as ameloblastoma, affects epithelial proliferation through an autocrine signaling, whereas angiogenic activity is mediated by a paracrine mechanism 7,21. However, in the only study describing VEGF-A expression in MO, it has been associated primarily with the angiogenic mechanisms of this tumor 7.
In the OM samples tested, high orosomucoid-1 immunoexpression was observed in tumor cells and ECM. This confirms the findings of the two studies that reported orosomucoid-1 overexpression in OM by immunohistochemistry and proteomics 7-22.
Macroscopically, OM appears as a highly viscous mucous mass, which, according to our results, could be partly due to the presence of orosomucoid-1 (which is a mucoprotein) in the ECM. It is widely accepted that the structural viscosity of the OM enables it to infiltrate bone and invade 7. In turn, García Muñoz et al. (2012) proposed that overexpression of orosomucoid-1 in OM could play a major role in tumor cell growth and invasion potential by inhibiting antitumor immune response 22.
Orosomucoid-1 has been shown to participate in VEGF-A regulation and induction 23-24. Thus, overexpression of these proteins in OM in both, tumor and endothelial cells, could indicate an interaction between orosomucoid-1 and VEGF-A in this tumor, suggesting a collaborative proangiogenic role 7.
In this study, most cases expressed high immunopositivity for mast cell tryptase (96.70%) in tumor cells, mast cells, and the ECM. A previous study including seven cases of OM reported no expression of mast cell tryptase; however, the sample size may not be representative 25. Another study conducted in a larger sample (62 cases) found mast cell tryptase in 72.6% of OM and it was mainly located in mast cells, which have large amounts of active tryptase stored, than, when released, can degrade fibronectin (one of the components of the OM’s ECM). This suggests that, to some extent, ECM degradation could be mediated by the release of mast cell tryptase 9-26. Additionally, there is an association between the presence of mast cells and mast cell tryptase with bone resorption in odontogenic cysts 27, and although the incidence of mast cells in OM varies according to different studies, it has been described that these cells are frequently distributed adjacent to residual bone trabeculae 28. These findings suggest that ECM degradation and bone remodeling processes via tryptase contribute significantly to the invasion potential of OM (27, -28).
The imbalance between apoptosis mechanisms and cell proliferation accounts for significant tumorigenic molecular alterations in various neoplasms.
In this study we evaluated p53, Bcl-2 and Ki-67, which are proteins related to apoptotic, anti-apoptotic and cell proliferation processes respectively. The apoptosis induced by p53 has been shown to be blocked by Bcl-2 29. Both markers, p53 and Bcl-2, showed a low immunoexpression in most OM, which is consistent with previous reports 9,30. Likewise, Ki-67 showed a low proliferation rate in our cases, which is consistent with the work of various authors 9,30. These results could indicate that, due to the low level of anti-apoptotic and proliferative activity in this tumor, these would not be the main mechanisms associated with the growth potential and aggressiveness of OM.
Conclusions
CK19 and CD138 labelling is useful for detecting odontogenic epithelium in OM. The high expression of orosomucoid-1 in the ECM may indicate it is a structural component that contributes to its viscosity and, therefore, facilitates invasion. Orosomucoid-1 also showed a VEGF-A-like pattern in tumor and endothelial cells, suggesting proangiogenic collaborative activity, possibly involved in tumor development. Anti-apoptotic and proliferative activity do not seem to be crucial mechanisms in the aggressive behavior of OM. However, ECM degradation and bone resorption mediated by mast cell tryptase could be significant in the high invasive potential of OM.
REFERENCES
1. El-Naggar Chan JKC, Grandis JR, Takata T, Slootweg P, editors. WHO classification of Head and Neck Tumours. Chapter 8: Odontogenic and maxilofacial bone tumours. 4th edition, IARC: Lyon 2017, p.205-260. [ Links ]
2. Mosqueda-Taylor A, Ledesma-Montes C, Caballero-Sandoval S, Portilla-Robertson J, Ruíz-Godoy RLM, Meneses-García A. Odontogenic tumors in México. A collaborative retrospective study of 349 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997; 84 (6): 672-5. [ Links ]
3. Wright JM, Soluk-Tekkesin M. Odontogenic tumors: where are we in 2017? J Istanb Univ Fac Dent. 2017; 51 (3 Suppl 1): s10-30. [ Links ]
4. Gonzalez-Galvan MC, Mosqueda-Taylor A, Bologna-Molina R, Setien-Olarra A, Marichalar-Mendia X, Aguirre-Urizar JM. Evaluation of the osteoclastogenic process associated with RANK / RANK-L / OPG in odontogenic myxomas. Med Oral Patol Oral Cir Bucal. 2018; 23 (3): e315-9. [ Links ]
5. Kanitkar S, Kamat M, Tamagond S, Vareakr A, Datar U. Peripheral odontogenic myxoma in a 12-year-old girl: a rare entity. J Korean Assoc Oral Maxillofac Sur. 2017;43: 178.81. doi: 10.5125/jkaoms.2017.43.3.178 [ Links ]
6. Chrcanovic BR, Gomez RS. Odontogenic myxoma: an updated analysis of 1,692 cases reported in the literature. Oral Dis. 2019; 25: 676-83. doi: 10.1111/odi.12875 [ Links ]
7. Bologna-Molina R, Mosqueda-Taylor A, Dominguez-Malagon H, Salazar-Rodriguez S, Tapia G, Gonzalez-Gonzalez R, Molina-Frechero N. Immunolocalization of VEGF-A and orosomucoid-1 in odontogenic myxoma. Rom J Morphol Embryol. 2015; 56 (2): 465-73. [ Links ]
8. Weidner N, Semple JP, Welch WR, Folkman J. Tumor angiogenesis and metastasis - correlation in invasive breast carcinoma. N Engl J Med. 1991; 32: 1-8. [ Links ]
9. Martínez-Mata G, Mosqueda-Taylor A, Carlos-Bregni R, Paes de Almeida O, Contreras-Vidaurre E, Vargas PA, Cano-Valdéz AM, Domínguez-Malagón H. Odontogenic myxoma: clinico-pathological, immunohistochemical and ultrastructural findings of a multicentric series. Oral Oncol. 2007; 44: 601-7. doi:10.1016/j.oraloncology 2007.08.009. [ Links ]
10. Lombardi T, Lock C, Samson J, Odell EW. S100, alpha-smooth muscle actin and cytokeratin19 immunohistochemistry in odontogenic and soft tissue myxomas. J Clin Pathol. 1995; 48: 759-62. [ Links ]
11. Etemad-Moghadam S, Alaeddinni M. A comparative study of syndecan-1 expression in different odontogenic tumors. J Oral Biol Craniofac Res. 2017; 7: 23-6. doi: 10.1016/j.jobcr.2016.11.001 [ Links ]
12. Bologna-Molina R, Salazar-Rodríguez S, Bedoya-Borella AM, Carreon-Burciaga RG, Tapia-Repetto G, Molina-Frechero N. A histopathological and immunohistochemical analysis of ameloblastic fibrodentinoma. Case Rep Pathol. 2013; 2013:604560. doi: 10.1155/2013/604560 [ Links ]
13. Bologna-Molina R, Mosqueda-Taylor A, López-Corella E, Paes de Almeida O, Carrasco-Daza D, Farfán-Morales JE, Molina-Frechero N, Damián-Matsumura P. Comparative expression of syndecan-1 and Ki-67 in peripheral and desmoplastic ameloblastomas and ameloblastic carcinoma. Pathol Int. 2009; 59: 229-33. [ Links ]
14. Manne RK, Kumar VS, Venkata Sarath P, Anumula L, Mundlapudi S, Tanikonda R. Odontogenic myxoma of the mandible. Case Rep Dent. 2012; 2012: 214704. doi: 10.1155/2012/214704 [ Links ]
15. Alaeddini M, Etemad-Moghadam S, Baghaii F. Comparative expression of calretinin in selected odontogenic tumours: a possible relationship to histogenesis. Histopathology. 2008; 52 (3): 299-304. [ Links ]
16. Terracciano LM, Mhawech P, Suess K, D'Armiento M, Lehmann FS, Jundt G, Moch H, Sauter G, Mihatsch MJ. Calretinin as a marker for cardic myxoma. Diagnostic and histogenetic considerations. Am J Clin Pathol. 2000; 114 (5): 754-9. [ Links ]
17. Mistry D, Altini M, Coleman HG, Ali H, Maiorano E. The spatial and temporal expression of calretinin in developing rat molars (Rattus norvegicus). Arch Oral Biol. 2001; 46 (10): 973-81. [ Links ]
18. Altini M, Coleman H, Doglioni C, Favia G, Maiorano E. Calretinin expression in ameloblastomas. Histopatholohy. 2000; 37 (1): 27-32. [ Links ]
19. Seifi S, Shafaie S, Ghadiri S. Microvessel density in follicular cysts, keratocystic odontogenic tumours and ameloblastomas. Asian Pacific J Cancer Prev. 2001; 12 (2): 351-56. [ Links ]
20. Pereira T, Dodal S, Tamgadge A, Bhalerao S, Tamgadge S. Quantitative evaluation of microvessel density using CD34 in clinical variants of ameloblastoma: An immunohistochemical study. J Oral Maxillofac Pathol. 2016; 20 (1): 51-8. Doi: 10.4103/0973-029X.180929 [ Links ]
21. Gupta B, Chandra S, Singh A, Sah K, Raj V, Gupta V. The role of vascular endothelial growth factor in proliferation of odontogenic cysts and tumors: An immunohistochemical study. Dent Res J. 2016; 13 (3): 256-63. [ Links ]
22. García-Muñoz A, Rodríguez MA, Bologna-Molina R, Cázares-Raga FE, Hernández-Hernández FC, Farfán-Morales JE, Trujillo JJ, Licéaga-Escalera C, Mendoza-Hernández G. The orosomucoid 1 protein (a1 acid glycoprotein) is overexpressed in odontogenic myxoma. Proteome Sci. 2012; 10 (1): 49. [ Links ]
23. Irmak S, Oliveira-Ferrer L, Singer BB, Ergun S, Tilki D. Pro-angiogenic properties of orosomucoid (ORM). Exp Cell Res. 2009; 315 (18): 3201-09. [ Links ]
24. Ligresti G, Aplin AC, Dunn BE, Morishita A, Nicosia RF. The acute phase reactant orosomucoid-1 is a bimodal regulator of angiogenesis with time- and context dependent inhibitory and stimulatory properties. Plos One. 2012; 7 (8): e41387. doi: 10.1371/journal.pone.0041387 [ Links ]
25. Kouhsoltani M, Halimi M, Dibazar S. A positive correlation between immunohistochemical expression of CD31 and mast cell tryptase in odontogenic tumor. Pol J Pathol. 2015; 66 (2): 170-5. [ Links ]
26. Fajardo I, Pejler G. Human mast cell ß-tryptase is a gelatinase. J Immunol. 2003; 171 (3): 1493-9. [ Links ]
27. Huang S, Lu F, Chen Y, Huang B, Liu M. Mast cell degranulation in human periodontitis. J Periodontol. 2013; 84 (2): 248-55. [ Links ]
28. De Assis Caldas Pereira F, Araújo Silva Gurgel CA, Ramos EA, Vidal MT, Pinheiro AL, Jurisic V, Sales CB, Cury PR, dos Santos JN. Distribution of mast cell in benign odontogenic tumors. Tumor Biol. 2012; 33 (2): 455-61. [ Links ]
29. Iezzi G, Piatelli A, Rubini C, Artese L, Fiorini M, Carinci F. MIB-1, Bcl-2 and p53 in odontogenic myxomas of the jaws. Acta Otorhinolaryngol Ital. 2007; 27 (5): 237-42. [ Links ]
30. Bast B, Pogrel MA, Regezi JA. The expression of apoptotic proteins and matrix metalloproteinases in odontogenic myxomas. J Oral Maxillofac Surg. 2003; 61 (12): 1463-6. [ Links ]
Authorship contribution: 1. Conception and design of study 2. Acquisition of data 3. Data analysis 4. Discussion of results 5. Drafting of the manuscript 6. Approval of the final version of the manuscript ZGH, CSR and RBM have contributed in 1,2,3,4,5,6. GVB and VPP have contributed in 3,4,5,6. ES has contributed in 4,5,6. OTM has contributed in 1,2,3.
Received: July 25, 2019; Accepted: December 09, 2019










 texto em
texto em