Services on Demand
Journal
Article
Related links
Share
Odontoestomatología
Print version ISSN 0797-0374On-line version ISSN 1688-9339
Odontoestomatología vol.21 no.34 Montevideo Dec. 2019 Epub Dec 01, 2019
https://doi.org/10.22592/ode2019n34a5
Update
Bioactive glasses in restorative dentistry
1Odontología Restauradora Integral, Facultad de Odontología, Universidad de la República, Montevideo, Uruguay. migarchitorena@gmail.com
Bioactive glasses (BG) are ceramic materials whose chemical composition allows them to induce and conduct tissue mineralization. As these glasses can be obtained with the sol-gel method and in nanometric particle sizes, their indication has been extended and enhanced.
The antibacterial properties of BG are outstanding: they are possible given the release of ions, which alkalinizes the medium, acting on the bacterial colonies.
The medical and dental applications of these materials are wide, with an emphasis on bone regeneration, remineralization of hard dental tissues and treatment of hypersensitivity. However, as they are materials with an amorphous chemical structure, their mechanical properties are not good, this being their main limitation for clinical application in restorative dentistry. In this sense, scientific research has focused on determining the possibility of including BG in various dental materials as a way to combine bioactivity with appropriate mechanical properties.
So far, it has not been possible to determine the proportion and methodology necessary to include BG in dental materials without altering their clinical behavior, which is why further research is necessary.
Keywords: bioactive glass; restorative dentistry; remineralization
Los vidrios bioactivos (VB) son materiales cerámicos con una composición química tal que poseen la propiedad de inducir y conducir la mineralización de los tejidos. La obtención de estos vidrios por medio del método sol-gel y la posibilidad de obtener tamaño nanométrico de partícula, han ampliado y potenciado las indicaciones de estos materiales.
Las propiedades antibacterianas de los VB son una característica sobresaliente; es debida a la liberación de iones que alcaliniza el medio, actuando sobre las colonias bacterianas.
Las aplicaciones médicas y odontológicas de estos materiales son muy amplias, destacándose la regeneración ósea, la remineralización de los tejidos duros dentarios y el tratamiento de la hipersensibilidad. Sin embargo, por tratarse de materiales con estructura química amorfa, sus propiedades mecánicas no son buenas, siendo esta característica su principal limitación para la aplicación clínica en el área de la odontología restauradora. En este sentido las investigaciones científicas se han enfocado en determinar la posibilidad de incorporar VB a diversos materiales dentales como forma de combinar su bioactividad con propiedades mecánicas apropiadas.
Hasta el momento no se ha logrado determinar la proporción y la metodología para incorporar VB en los materiales dentales sin alterar su comportamiento clínico, por lo que son necesarias más investigaciones.
Palabras clave: vidrios bioactivos; odontología restauradora; remineralización
Os vidros bioativos (VB) são materiais cerâmicos com uma composição química tal que eles possuem a propriedade de induzir e conduzir a mineralização dos tecidos. A obtenção desses vidros por meio do método sol-gel e a possibilidade de obtenção de partículas nanométricas ampliaram e reforçaram as indicações desses materiais. As propriedades antibacterianas doVB são uma característica marcante; é devido à liberação de íons que alcaliniza o meio, atuando nas colônias bacterianas. As aplicações médicas e odontológicas desses materiais são muito amplas, destacando-se a regeneração óssea, a remineralização dos tecidos duros e o tratamento da hipersensibilidade. No entanto, por serem materiais com estrutura química amorfa, suas propriedades mecânicas não são boas, sendo essa a sua principal limitação para aplicação clínica na área de odontologia restauradora. Nesse sentido, a pesquisa científica tem se concentrado em determinar a possibilidade de incorporar VB em vários materiais odontológicos, como forma de combinar sua bioatividade com propriedades mecânicas apropriadas.
Até agora, não foi possível determinar a proporção e metodologia para incorporar VB em materiais odontológicos sem alterar seu comportamento clínico, razão pela qual mais pesquisas são necessárias.
Palavras-chave: vidro bioactivo; odontologia restorativa; remineralização
Introduction
Remineralization is a process which involves restoring lost mineral ions to the dental structure, which enables the strengthening and functionality of the crystalline structure1.
Bones and teeth are incredibly complex organs, with a combination of different hard tissues (trabecular and compact bone, tooth enamel, dentin, dental cementum) and soft tissues (bone marrow, dental pulp, periodontal ligament). They have a unique hierarchical structure, with a combination of complex phenomena, such as biomolecular interactions, nutrient exchange or fluid transport2.
The interaction of materials with dental tissues is determined by a series of factors such as composition, particle size, the chemistry of the released elements and the ability of tissues to respond to these agents3. Today's dentistry aims to repair damaged tissues and restore them to their natural condition, instead of replacing them with inert synthetic materials. Materials science not only studies the potential toxicity of materials but mainly focuses on the specific tissue responses they can trigger.
New materials, among them BG, have emerged, which involve the development of techniques to remineralize dental structures and, in recent years, a new paradigm has been proposed in healthcare: regenerative dentistry. It proposes repairing damaged tissues using mechanisms similar to those used by the body to renew cell populations. This approach requires using porous biomaterials, called scaffolds, which enable and favor the growth and organization of live tissue from cell cultures and appropriate biochemical factors, which induce and promote the regeneration of damaged tissue. This poses new challenges related to the development of appropriate three-dimensional scaffolds for cells to grow, proliferate and develop their specific function.BG can be used for this purpose since they meet the necessary requirements, such as osteoinduction, osteoconduction, biodegradability, biocompatibility, radiopacity, appropriate mechanical properties, ease of handling and sterilization4. The addition of these glasses to other restorative dental materials is also being researched to provide bioactive and antimicrobial properties that could improve the prognosis for treatments.
The aim of this review is to study BG further and to determine the possibility of including them in various restorative dental materials.
Methodology
The literature review was conducted in PubMed, Timbó and Scielo. The inclusion criterion was the date of the papers, including those published since 2000 (except for articles by Larry Hench which, due to their relevance, were included despite having been published before 2000) both in English and in Spanish.
Development
The conceptual and technical evolution of these materials has progressed from avoiding damage, by using inert materials, then biocompatible materials and finally regenerative materials5-7. These replace dental tissues by applying mechanisms which are similar to those occurring in the body8. Bioactive materials are those which elicit a biological response in tissues resulting in a strong chemical bond between the material and hard and soft tissues2,6,9.
An ideal bioactive material should10:
a)be bactericidal
b)be bacteriostatic
c)be sterile
d)stimulate dentin formation
e)preserve pulp vitality.
In 1969, Larry Hench developed bioactive glasses. Looking for a material that could bind to bone, he found a composition containing 45% by weight of silicon dioxide (SiO2), 24.5% by weight of sodium oxide (Na2O), 24.5% by weight of calcium oxide (CaO), and 6% by weight of phosphorus oxide (P2O5), commercialized under the name Bioglass® 45S5 starting in 19852,11-12.
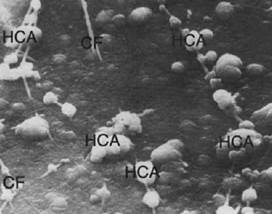
Fig. 1: The image shows the formation of hydroxyapatite on the surface of the material and closely associated with collagen fibers
The first paper on BG was published in 1971 and showed in vitro and in vivo results of the bond between the bone and the BG. This chemical bond is extremely strong because of the binding to the collagen structure; however, this bond with organic structures wasn’t discovered until 198113 (Fig. 1).
Glass was originally obtained with the traditional processing method which required subjecting a mixture of reagents, at appropriate molecular concentrations, to high temperatures (above 1300oC), melting the oxides in a platinum crucible, and then quenching it. Quenching increases the viscosity, which causes glass to solidify. This method has several disadvantages, mainly due to the high temperatures used, which leads to a highly crystalline structure, which is considered to be insoluble in the physiological medium9,14-16.
Since 1991, this traditional method has been replaced by the sol-gel method, in which a chemical synthesis of silica precursors is used to form and assemble nanoparticles into a gel at room temperature17. This new method has significant advantages: it requires significantly lower temperature (600 to 700oC), the glasses obtained are more pure and homogeneous, the ability to better control particle size (enabling the production of nanoparticles), and it makes it possible to enhance bioactivity. Also, the cost is lower. Nanoporous structures (with 2- to 50-nm pores) can be generated using the sol-gel method, thus increasing surface area and, as a result, increasing bioactivity5,14-18. Porosity also means the glass can be used as a scaffold for tissue regeneration15 and to form capsules to carry enzymes, antibiotics and antigens2,5,16.
Hench classifies bioactive materials into two classes: a) those in which bioactivity leads to both induction and production as a consequence of rapid reactions on the surface of the material; b) those in which only conduction is present because surface reactions are slower 11. Class A bioactivity gives rise to both intracellular and extracellular responses, which bind the material to the hard and soft tissues, whereas class B only induces an extracellular response19.
Bioactive glasses (BG) are ceramic materials classified as class A bioactive materials9.
When in contact with body fluids, chemical reactions occur on the surface leading to the formation of a layer of hydroxy carbonate apatite which favors its biocompatibility and integration into bones and hard dental tissues4,20. When BG are in physiological environments, that is, in solution, osteoinduction and the production of growth factors are activated, which results in the formation of bone with the same characteristics as healthy bone7-13. There is evidence, both in vivo and in vitro, that dissolution products leached from BG, that is, separated from the structure of the material when in a physiological environment, have an angiogenic effect. Direct stimulation by dissolution products increases growth factors, such as vascular endothelial growth factor (VEGF) and the basic fibroblast growth factor (bFGF) in fibroblast, and also regulates the expression of their respective receptors21). Moreover, the increase in pH associated with the dissolution-precipitation of BG affects cellular processes and is connected to an increase in metabolic activity and the proliferation rate in eukaryotic cells21-22.
When in aqueous solutions or body fluids, the surface of a BG implant turns into a layer of silica gel rich in calcium oxide and phosphorus oxide, which then mineralizes into hydroxycarbonate within hours23. In glasses with high levels of bioactivity, such as Bioglass® 45S5, the first stages of reactions occur very rapidly and are completed within 24 hours14.
BG have been proven to be effective against oral bacteria, such as Streptococcus sanguis, Streptococcus mutans and Actinomyces viscosus. An in vitro study showed a reduction in bacterial viability following exposure to the glass for one hour. This antibacterial effect increases after three hours24.
The current applications of bioactive glasses are manifold: they are used for bone replacement, periodontal defect repair, maxillofacial reconstruction, alveolar ridge augmentation, treatment of tumors, bioactive coatings on metallic substrates (e.g., in dental implants), as a treatment for dental sensitivity, tooth remineralization, cranial repair, percutaneous access devices, fibula repair, orthopedics and ENT16.
Discussion
The weak mechanical properties of BG, mainly its low fracture resistance, limit their applications. For this reason, combinations of BG and various materials that act as vehicles for its clinical application are sought25. Likewise, the possibility of incorporating BG into restorative materials and adhesive systems to provide them with antibacterial characteristics and promote remineralization could be an alternative to conservative treatments in restorative dentistry. Altering dental materials with BG is one of the most exciting applications in dentistry since a combination of these materials could generate a response in the marginal area of restorations, which is the most critical and sensitive to the recurrence of caries. It could also create an attachment area between the restorative material and gingival tissues in cases of full-crown restorations, since the material behaves like enamel9,26.
However, studying these combined materials poses multiple challenges. First, obtaining homogeneous materials is difficult, as they usually have pores as a result of the production technique used. Furthermore, tests to assess the mechanical behavior do not, for the most part, replicate oral conditions. The best way to predict the clinical behavior of materials is to design tests which replicate, as much as possible, clinical situations, by using in vitro specimens of sizes and shapes similar to those used clinically and following standardized clinical and laboratory protocols 27. Studies aim to determine the benefits BG bring to dental materials, and also to observe the potential alterations they can introduce in those materials, in connection with their optical properties, mechanical properties, hardening, etc.
As for the benefits provided by BG, the antimicrobial and remineralization potential has been studied. A recent in vitro study has shown antibacterial effectiveness in resin with a 15% addition of BG28. Bacterial penetration into marginal gaps was found to be much smaller in the samples in which the experimental material was used, in comparison to the control material (Fig. 2). This antimicrobial benefit is in addition to the ability of BG to remineralize dental structures; therefore, it is an up-and-coming alternative in restorative dentistry. Nevertheless, materials with these characteristics are, unfortunately, not yet commercially available.
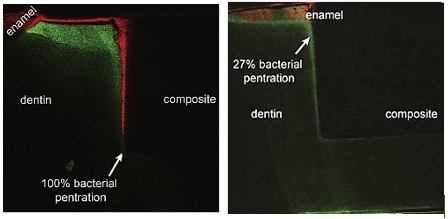
Fig. 2: Images show (by fluorescence) the difference in bacterial penetration in the dentin-resin and dentin-resin/BG interface (Khvostenko 2015)
Regarding the remineralization of hard dental tissues, the effect of BG was studied both in enamel and on dentin.
BG appear as a promising alternative to the remineralization of enamel, and they have been modified with fluoride ions to enhance their remineralizing power by forming fluorapatite29.
It has also been observed that BG adhere firmly to dentin, and that the ions in the glass penetrate the dentin tissue, while the glass surface in contact with the dentin is altered, which leads to apatite formation at the interface 30. An in vitro study to examine the mineralization of dentin with BG showed mineral nucleation and precipitation in the tissue. 31. However, it has yet to be confirmed whether this net remineralization is also functional remineralization which restores the mechanical properties to tissue22 since it has not been possible to restore mechanical properties in all the studies analyzed22,32-33.
In an in vitro study using glass ionomer enhanced with resin and BV, the increase in the flexural strength of demineralized dentin was confirmed34. The study showed a 10% increase in flexural strength in healthy dentin and a 20% increase in demineralized dentin. The authors assume that the difference between these and previous studies could be that different dentin demineralization methods were used: whereas EDTA was used in previous studies33, Khoroushi et al. used a mixture of calcium chloride, monopotassium phosphate and acetic acid. According to the authors, by completely destroying the collagen matrix, the dentin produced by EDTA is more deteriorated than what is observed in natural carious lesions, which would make it impossible to adequately remineralize it and, therefore, to restore the flexural strength. The attachment of BG to the dentin structure is mostly due to its connection to the collagen matrix. According to the authors, the demineralizing substance used in this study helps to replicate the clinical conditions of the dentin lesion faithfully by selectively eliminating the inorganic contents, without affecting the organic structure34.
A recent in vitro study showed the mechanical recovery of dentin, in terms of the modulus of elasticity and hardness. This recovery is explained by the association between the newly formed minerals and the organic matrix 35.
NovaMin® (NovaMin Technology, GlaxoSmithKline, Florida, UK), a glass with very fine particles of approximately 18 μm, was developed as a remineralizing and desensitizing agent for toothpaste2,15,18-19. This particulate glass also has antiplaque, antigingivitis, desensitizing and regenerating properties36.
The use of BG as a desensitizing element in dental prophylaxis (OSspray Ltd., UK) has been studied through a clinical assessment of the effectiveness of sodium bicarbonate powder and powder containing BG. A higher statistical and clinical stain removal efficacy was observed with the powder containing BG, in addition to more comfort for patients by reducing the sensitivity generated during the procedure. This occurs because BG particles seal the tubules. An important aspect of this desensitizing effect is that is long-lasting, due to the fact that it not only involves the mechanical occlusion of the tubules but also the deposition of HA as a result of glass particle reactions. This HA is chemically and structurally similar to natural structures15,37. Replacing abrasive materials, such as alumina, with BG in dental prophylaxis has the benefit of allowing for remineralization, causing less abrasiveness, and not having any adverse respiratory effects15,38.
The bond between dental materials and dentin is still a challenge due to the difficulties in avoiding hydrolysis, which occurs in adhesives after some time. Hydrolysis is caused by the presence of moisture in tissues, which affects the mineral-depleted collagen structure. Self-etch and total-etch techniques are not able to replace the water present in the intrafibrillar and extrafibrillar spaces of the collagen matrix39. This is why alternative adhesive techniques for dentin, resulting from the development of more interactive materials, are necessary to overcome these challenges30. One alternative for preventing hydrolysis in the resin-dentin interface is to develop a material which can induce the remineralization of denuded collagen, thus avoiding protein degradation and, consequently, adhesive failure39.
The possibility of developing adhesive systems with the addition of BG is being explored, testing whether the adhesive strength is affected35. The behavior of two adhesive systems, one containing BG 45S5 and the other one containing zinc-modified BG (45S6) on demineralized dentin specimens was analyzed in an in vitro study. There was a statistically significant increase in the mechanical properties (hardness and modulus of elasticity) of the tissue, which was due to the remineralization achieved35.
Studies have reached mixed results as for the potential alterations that adding BG can cause to dental materials. According to researchers, two aspects are causes of concern and, therefore, the object of numerous studies: on the one hand, the lack of bond between the BG and the resin matrix and, on the other hand, the release of ions that is characteristic of bioactivity, both of which cause the mechanical alteration observed in resin materials containing BG40.
The addition of BG to a conventional GI was analyzed in 1999, and it was determined that the setting time is “basically” not altered41.
A study was conducted in 2003 to evaluate the effect that adding BG to a resin-modified GI material could have on the setting time and mechanism and on the mechanical properties of the material. It was observed that the setting time increases and the compressive strength decreases42.
The compressive strength, the modulus of elasticity and Vickers hardness were studied in both conventional GI and resin-modified GI to which BG was added43. It was found that mechanical properties are altered, to a certain extent, which led to the suggestion of restricting the use of these combined materials to clinical cases which can benefit from their bioactive properties and which do not require a great load-bearing capacity, for example, for pulp protection or as an endodontic sealant.
In 2008, BG was added to a commercially available glass ionomer (Fuji 1, GC, Japan)44. Micrometric glass powder was used at proportions of 10 to 30%. The behavior of the material, with respect to setting time, tensile strength and bioactivity, was analyzed in vitro. The setting time was extended as the proportion of BG increased; however, tensile strength was not affected44.
The possibility of incorporating a fluoride-contiaining BG on a ratio of 12 to 15% by weight, to a composite material to provide fluoride and calcium to the site from a single source, as well as the possibility of recharging the material with fluoride by exposing it to a 5,000 ppm fluoride solution has been studied. It could be demonstrated that by using BG obtained by sol-gel method, it is possible to quickly release ions and recharge the composite material with fluoride, without altering its mechanical properties. This is thanks to the larger reactive surface area generated in BG produced using the sol-gel method45. This 15% portion of BG in the resin material has proven to be effective in providing the material with bioactive and antibacterial properties, without altering its mechanical properties40.
A study was conducted in 2011 to determine the mechanical properties, especially the flexural strength, of a restorative material combining ceramic and BG. Leucite porcelain (k) was used at between 50 and 70%, and it was found that it is possible to obtain a material with appropriate mechanical properties without losing bioactivity. Mechanical properties improve as the content of ceramic in the material increases46. Later studies concluded, despite the limitations of in vitro research, that the ideal composition for good mechanical properties without losing bioactivity is 20% of BG and 80% ceramic27. An interesting aspect of this composite material would be the potential formation of a gingival attachment around full-crown restorations, facilitated by the bioactive behavior and its composition, which is similar to tooth enamel. This attachment would seal the tooth-restoration interface, thus eliminating the potential for dissolution and degradation for the cement used for fixation, recurrence of caries and a possible restoration failure46-47.
Final remarks
The remineralization of dental structures with bioactive materials is a field in which restorative dentistry has evolved hand in hand with minimal intervention dentistry. Although they were developed decades ago, BG have gained new momentum in recent years, mostly thanks to the scientific advances which have made it possible to control production methods and particle size.
However, it is still necessary to define some significant aspects, such as synthesis techniques, ideal percent composition, etc., which will allow for the application of BG in restorative materials. Further research is necessary to incorporate the bioactive elements into restorative materials and combine the properties of both phases.
Given the potential of these materials, the utopian goal of regenerating dental tissue might not be so far away.
REFERENCES
1. Ferracane JL, Cooper PR, Smith AJ. Can interaction of materials with the dentin-pulp complex contribute to dentin regeneration? Odontology 2010; 98 (1): 2-14 [ Links ]
2. Krishnan V, Lakshmi T. Bioglass: A novel biocompatible innovation. J Adv Pharm Tech Res. 2013; 4 (2): 78-83 [ Links ]
3. Salinas AJ, Vallet-regí M. The Sol-Gel Production of Bioceramics. Key Engineering Materials. Trans Tech Publications, 2009; 391:141-158 [ Links ]
4. Sarin S, Rekhi A. Bioactive glass: A potential next generation biomaterial. SRM J Res Den Sci. 2016; 7 (1): 27 [ Links ]
5. Saqib A, Imran F, Kefi I. A review of the effect of various ions on the properties and the clinical applications of novel bioactive glasses in medicine and dentistry. The Saudi Dental Journal. 2014;26, 1-5 [ Links ]
6. López Piriz R. Vidrios bioactivos en Odontología. Gaceta dental. 2016; 281: 104-22 [ Links ]
7. Fernando D, Attik N, Pradelle-Plasse N, Jackson P, Grosgogeat B, Colon P. Bioactive glass for dentin remineralization: A systematic review. Materials Science and Engineering. 2017. [ Links ]
8. Kaur G, Pandey O P, Singh K, Homa D, Scott, B, Pickrell G. A review of bioactive glasses: their structure, properties, fabrication and apatite formation. J Biomed Mater Res A. 2014;102 (1): 254-74. [ Links ]
9. Sonarkar S, Purba R. Bioactive materials in conservative dentistry. Inter J Contemp Dent Med Rev. 2015: 1-4. [ Links ]
10. Sepúlveda Rebaudo, G . Evaluación de la bioactividad del cemento biodentine modificado con nanopartículas de vidrio bioactivo. Universidad de Chile, Facultad de Odontología. 2015. [ Links ]
11. Badami V, Ahuja B. Biosmart Materials: Breaking New Ground in Dentistry. Scientific World J. 2014; 2014: 986912. doi:10.1155/2014/986912 [ Links ]
12. Abbasi Z, Bahrololoum M E, Bagheri R, Shariat M H. Characterization of the bioactive and mechanical behavior of dental ceramic/sol-gel derived bioactive glass mixtures. J of the mechanical behavior of biomedical materials. 2016; 54: 115-22. [ Links ]
13. Polini A, Bai H, Tomsia A. Dental applications of nanostructured bioactive glass and its Composites. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2014; 5 (4): 399-410 [ Links ]
14. Hench L. The story of Bioglass. J Mater Sci: Mater Med.2006; 17: 967-978 . [ Links ]
15. Khoroushi, M., Keshani, F. A review of glass-ionomers: From conventional glass-ionomer to bioactive glass-ionomer. Dental research journal. 2013; 10 (4): 411-420. [ Links ]
16. Narayana S, Deepa VK, Ahamed S, Sathish ES, Meyappan R, Kumar S. Remineralization efficiency of bioactive glass on artificially induced carious lesion an in-vitro study. Journal of Indian Society of Pedodontics and Preventive Dentistry 2014; 32(1): 19-25. [ Links ]
17. Hench, LL. Bioceramics: from concept to clinic. J American Cer Soc. 1991; 74 (7): 1487-1510. [ Links ]
18. Salonen JI, Arjasamaa M, Tuominen U, Behbehani MJ, Zaatar EI. Bioactive glass in dentistry. J Minim Interv Dent. 2009; 2 (4): 208-18. [ Links ]
19. Jones JR. Review of bioactive glass: from Hench to hybrids. Acta biomaterialia. 2013;9(1):4457-4486. [ Links ]
20. Aguiar H, Serra J, González P. Los vidrios bioactivos en el mundo de los biomateriales. An. Quím. 2011; 107 (3): 237-42. [ Links ]
21. Hench L. Chronology of Bioactive Glass Development and Clinical Applications. New J Glass Ceramics. 2013; 3 (2): 67-73. [ Links ]
22. Sepulveda P, Jones JR, Hench LL. Characterization of melt-derived 45S5 and sol-gel-derived 58S bioactive glasses. J Biomed Mater Res. 2001; 58 (6): 734-40. [ Links ]
23. Allan I, Newman H, Wilson M. Antibacterial activity of particulate bioglass against supra and subgingival bacteria .Biomaterials. 2001; 22: 1683-87 [ Links ]
24. Abbasi Z, Bahrololoom ME, Shariat MH, Bagheri R. Bioactive Glasses in Dentistry: A Review. J Dent Biomater. 2015; 2 (1):1-9. Allan I, Newman H, Wilson M. Antibacterial activity of particulate Bioglass against supra and subgingival bacteria .Biomaterials. 2001; 22: 1683-87. [ Links ]
25. Stanciu GA, Stanciu SG, Sandulescu I, Savu B. Investigation of the hydroxyapatite growth on bioactive glass surface. J. Biomed. Pharm. Eng. 2007; (1): 34-9. [ Links ]
26. Goudouri OM, Kontonasaki E, Papadopoulou L, Manda M, Kavouras P, Triantafyllidis KS, Paraskevopoulos KM. An experimental bioactive dental ceramic for metal-ceramic restorations: Textural characteristics and investigation of the mechanical properties. J Mec Behavior Biomed Mater. 2017; 66: 95-103. [ Links ]
27. Matsuya S, Matsuya Y, Ohta M. Structure of bioactive glass and its application to glass ionomer cement. Dent Mater J. 1999;18 (2): 155-66 [ Links ]
28. Khvostenko D, Hilton T J, Ferracane J L, Mitchell J C, Kruzic J J. Bioactive glass fillers reduce bacterial penetration into marginal gaps for composite restorations. Dent Mater. 2015; 32 (1): 73-81. [ Links ]
29. Brauer DS, Karpukhina N, O'Donnell MD, Law RV, Hill RG. Fluoride-containing bioactive glasses: effect of glass design and structure on degradation, pH and apatite formation in simulated body fluid. Acta Biomater. 2010; 6 (8): 3275-82. [ Links ]
30. Efflandt SE, Magne P, Douglas WH, Francis LF. Interaction between bioactive glasses and human dentin. Journal of Materials Science: Materials in Medicine. 2002; 13 (6): 557-65. [ Links ]
31. Forsback AP, Areva S, Salonen J. Mineralization of dentin induced by treatment with bioactive glass S53P4 in vitro. Acta Odontologica Scandinavica. 2004; 62 (1): 14-20. [ Links ]
32. Hannig M, Hannig C. Nanomaterials in preventive dentistry. Nat Nanotechnol. 2010; 5: 565-9. [ Links ]
33. Vollenweider M, Brunner TJ, Knecht S, Grass RN, Zehnder M, Imfeld T, Stark WJ. Remineralization of human dentin using ultrafine bioactive glass particles. Acta Biomater. 2007; 3: 936-43. [ Links ]
34. Khoroushi M, Mousavinasab SM, Keshani F,Hashemi S. Effect of resin-modified glass ionomer containing bioactive glass on the flexural strength and morphology of demineralized dentin. Oper Dent. 2013; 38 (2): E21-E30 [ Links ]
35 .Sauro S, Osorio R, Watson TF,Toledano M. Therapeutic effects of novel resin bonding systems containing bioactive glasses on mineral-depleted areas within thebonded- dentineinterface.J Mater Sci Mater Med .2012; 23: 1521-32 [ Links ]
36. Crovace M C, Souza M T, Chinaglia C R, Peitl O, Zanotto E D. Biosilicate(r)-A multipurpose, highly bioactive glass-ceramic. In vitro, in vivo and clinical trials. J Non-Crystalline Solids. 2016; 432: 90-110 [ Links ]
37. Kumar A, Singh S, Thumar G, Mengji A. Bioactive Glass Nanoparticles (NovaMin(r)) for Applications in Dentistry. J Dent Med. 2015;14 ( 8 ):30-5 [ Links ]
38. Banerjee A, Hajatdoost-Sani M, Farrell S, Thompson I. A clinical evaluation and comparison of bioactive glass and sodium bicarbonate air-polishing powders. J Dent. 2010; 38 (6): 475-79. [ Links ]
39. Farooq I, Imran Z, Farooq U, Leghari A, Ali H. Bioactive Glass: A Material for the Future. World J Dent. 2012; 3 (2):199-201. [ Links ]
40. Davis HB, Gwinner F, Mitchell JC, Ferracane JL. Ion release from, and fluoride recharge of a composite with a fluoride-containing bioactive glass. Dent Mater. 2014; 30 (10): 1187-1194. [ Links ]
41. Ana I D, Matsuya S, Ohta M, Ishikawa K. Effects of added bioactive glass on the setting and mechanical properties of resin-modified glass ionomer cement. Biomaterials. 2003; 24 (18): 3061-67. [ Links ]
42. Yli-Urpo H, Narhi M, Narhi T. Compuond changes and tooth mineralization effects of a glass ionomer cements containing bioactive glass (S53P4), an in vivo study. Biomaterials 2005; 26: 5934-41. [ Links ]
43. Choi J Y, Lee H H, Kim H W. Bioactive sol-gel glass added ionomer cement for the regeneration of tooth structure. J Mater Sci Mater Med 2008; 19 (10): 3287-94. [ Links ]
44. Khvostenko D, Mitchell JC, Hilton TJ, Ferracane JL, Kruzic JJ. Mechanical performance of novel bioactive glass containing dental restorative composites. Dent Mater. 2013; 29 (11): 1139-1148. [ Links ]
45. Bauer J, Carvalho E M, Carvalho C N, Meier M M, de Souza J P, de Carvalho R M, Loguercio A D. Development of a simplified etch-and-rinse adhesive containing niobiophosphate bioactive glass. Int J Adhesion Adhes 2016; 69: 110-14. [ Links ]
46. Goudouri OM, Kontonasaki E, Theocharidou A, Kantiranis N, Chatzistavrou X, Koidis P, Paraskevopoulos KM. Dental Ceramics/Bioactive Glass Composites: Characterization and Mechanical Properties Investigation. Bioceramics Development and Applications 2011;1:1-4. [ Links ]
47. Manda M, Goudouri OM, Papadopoulou L, Kantiranis N, Christofilos D, Triantafyllidis K, Koidis P. Material characterization and bioactivity evaluation of dental porcelain modified by bioactive glass. Ceramics Int 2012;38(7):5585-5596. [ Links ]
Received: September 05, 2018; Accepted: July 07, 2019











 text in
text in 



