Services on Demand
Journal
Article
Related links
Share
Odontoestomatología
Print version ISSN 0797-0374On-line version ISSN 1688-9339
Odontoestomatología vol.20 no.32 Montevideo Dec. 2018
https://doi.org/10.22592/ode2018n32a11
Research
Comparison of the oral health status of diabetic and non-diabetic Uruguayan children aged 8-12
1 Facultad de Odontología. Universidad de la República, Uruguay
2 Consultor estadístico, Montevideo, Uruguay
3 Unidad de Diabetes. Centro Hospitalario Pereira Rossell, Montevideo, Uruguay
4 Facultad de Odontología. Universidad de la República, Uruguay
Diabetes mellitus is a chronic disorder that affects oral health; there are no data in Uruguay.
Method:
Observational, analytical case-control study where 86 children were evaluated and divided into two groups: DM1 Group, diabetic children aged 8-12 who go to Pereira Rossell Hospital Center without other systemic diseases and with no orthodontic treatment; Control group (CG): non-diabetic children in the same age group with public health care coverage, non-medicated and without orthodontic treatment. Variables: biofilm, dental caries, gingival bleeding.
Results:
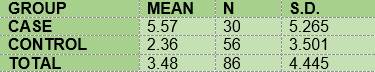
Biofilm was present in all subjects. O’Leary Index: DM1=71.48 and CG=89.81. Bleeding on probing: DM1=76.7% and CG=60.7% (GBI averages are 5.57 and 2.36 respectively and GBI mean: DM1=3.65 and CG=1.04. Caries: DM1=40.0% and CG=28.6% (DMFT average: DM1=1.223 and CG=1.0357).
Conclusions:
We found significant statistical differences in bleeding index between diabetic and non-diabetic subjects (Mann & Whitney test) but not in the caries variables. These results allow for a comparison with international studies.
Keywords: diabetes mellitus; oral health; prevalence; dental indexes.
La Diabetes Mellitus es una enfermedad crónica con repercusiones bucales; no existen datos en Uruguay.
Método:
Estudio caso-control, observacional, analítico. Se evaluaron 86 niños divididos en dos grupos: Grupo DM1: diabéticos de 8 a 12 años, que asisten al Centro Hospitalario Pereira Rossell, sin otra enfermedad sistémica ni tratamiento ortodóncico; Grupo control: no diabéticos de 8 a 12 años con asistencia en servicio médico público, no toman medicación y no cuenten con ortodoncia. Variables: biofilm, caries dental, sangrado gingival.
Resultados:
Todos los sujetos presentan biofilm. Media del índice de O’Leary: DM1=71,48 y Control=89,81. Sangrado al sondaje: DM1=76,7% y Control =60,7% (medias ISG: 5,57 y 2,36 respectivamente; mediana del ISG: DM1=3.65 y Control=1.04. Caries: DM1=40,0% y Control=28,6% (media CPOD: DM1=1,233 y Control=1,0357).
Conclusiones:
Existen diferencias estadísticamente significativas en el índice de sangrado entre diabéticos y no diabéticos (test de Mann y Whitney) pero no en las variables relativas a caries. Estos resultados posibilitan la comparación con estudios internacionales
Palabras clave: Diabetes Mellitus; Salud oral; Prevalencia; Índices odontológicos
Introduction
Diabetes mellitus (DM) is an endocrine metabolic non-communicable disease, which affects a high percentage of the world’s population; it is also one of the most frequent diseases among children and adolescents. The World Health Organization defines DM as a metabolic disorder with multiple aetiologies, characterized by chronic hyperglycemia and an alteration of the metabolism of carbohydrates, lipids and proteins, caused by a defect in the secretion of insulin, in its action, or both. In the long term, the consequences of DM involve the appearance of conditions in different organs or systems, such as the retina, the kidneys or the cardiovascular system 1. Diabetes mellitus occurs when the concentration of insulin is unsuitable to prevent hyperglycemia and its consequences. When there is insulin resistance, a metabolic status which reduces the effect of insulin on the tissues, it can be balanced by increasing the secretion of the β pancreatic cell; it has been proven that diabetes does not occur as long as hyperinsulinemia helps balance this situation (2.
In the 80s, it became clear that it was necessary to conduct rigorous epidemiological studies, in order to determine the extent and the impact of DM1. This led to several projects: the SearchStudy 3) in the United States of America and in Europe, the Diabetes Mondiale-DiaMond Project4 and the EURODIAB study5, all sponsored by the World Health Organization and aimed at keeping records based on population studies to monitor the trends of the disease in children and adolescents, its incidence and prevalence based on national and regional records, which have allowed for the standardization of definitions, protocolizing data collection and validation methods. The DiaMond project included over 110 records in 57 countries, and EURODIAB included records from 44 centers in 28 European countries. Additional studies reported by the International Diabetes Federation 6 have also become relevant to monitor DM trends around the world. These studies have provided evidence that the incidence of the onset of DM1 in childhood is rising in most countries around the world; the incidence of DM1 in children increases with age, peaking around puberty. Despite geographical, ethnic, and racial differences, the estimated annual growth rate in Europe is 3%, with a higher growth rate in younger people 5. Latin America is no exception; there has been a significant increase in the incidence of DM, which has called the attention of general physicians, medical and dental specialists. A nation-wide survey conducted in Uruguay in 20047 showed a prevalence of 6.4% of known diabetics, and 1.6% of not known diabetics: a total of 8.2%. The results were higher than in previous estimates, noting that 20% of the diabetics had not been diagnosed, and therefore did not know they suffered from the disease. Given that another 8% of the population is quite likely to suffer from diabetes, the prevalence in Uruguay might be around 16.2%, equivalent to around 534,000 Uruguayans suffering or at risk of developing DM. It is important to note that mortality from DM ranges from 12 to 14 for every 1,000 Uruguayans.
In 2018 8, the American Diabetes Association (ADA) stated that DM occurs when there is an unsuitable concentration of insulin to prevent hyperglycemia and its consequences. If there is insulin resistance, insulin levels may increase in order to compensate for it, and diabetes does not occur as long as the hyperinsulinemia compensates this situation. There are three main kinds of diabetes mellitus: type 1 (DM1), type 2 (DM2) and gestational diabetes 9-10. The most common diabetes mellitus among children and young people is type 1 (DM1). It is characterized by the destruction of the β pancreatic cells, which translates into an absolute insulin deficiency and a vital dependence on exogenous insulin. This is the case in over 80% of patients in the pediatric age group. It also appears in young adults, and it represents between 5% and 10% of all the DM cases at any age. Its highest incidence is observed among children under 15, which is why it is also called juvenile or insulin-dependent diabetes.
Clinical studies 11-15) have reported significant effects of diabetes mellitus on the oral cavity, affecting soft and hard tissues, such as caries, gingivitis/periodontitis and tooth loss. This study arises from the realization that Uruguay lacks knowledge and data about the relationship between diabetes mellitus and oral health in children, despite the fact that international scientific evidence recognizes diabetes mellitus as one of the chronic diseases with clear repercussions in the mouth.
Objective
To determine whether the oral health status in children suffering from diabetes mellitus type 1 is different from that of children who do not suffer from diabetes, by comparing the prevalence of dental caries and gingival alterations. The survey of diabetic children was conducted at the Diabetes Unit of the referral and counter-referral outpatient clinic of the Centro Hospitalario Pereira Rossell (CHPR) 16, the only pediatric hospital in the country which has a multiprofessional and interdisciplinary medical team made up of professors of the School of Medicine of Universidad de la República and professionals belonging to the National Health Service (ASSE) of the Ministry for Public Health (MSP). The control group was made up of students at State School No. 172, “José Martí”, located in Malvín neighborhood (Caldas 1921). Their socio-cultural context is similar to that of the patients treated at CHPR, and they also receive health care from state providers.
Methodology
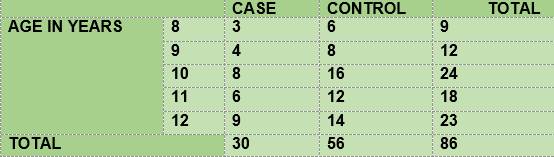
The study was designed as a case-control, observation-based and analytical study. The case group included all the diabetic children seen within the period from March to December, 2017, aged 8-12. The control group was made up of non-diabetic children aged 8-12 at the time of the test. Two controls of the same age were taken for each case. This is therefore a convenience sample (Table 1).
The distribution of subjects according to gender in the diabetic and non-diabetic groups was balanced: 15 females and 15 males in the group of diabetics, and 29 females and 27 males in the group of non-diabetics. The vast majority of the total number of subjects (91.7%) had received some kind of dental care. The mean of the Body Mass Index was 19.47 for diabetic children and 19.54 for non-diabetic children.
Two groups were evaluated:
GROUP DM1 (CASE). Inclusion criteria: Type 1 diabetic children aged 8-12, attending a state health care center after two or more years of the onset of diabetes. Exclusion criteria: Children with other systemic conditions with orthodontic treatment, who do not sign the consent form.
GROUP 2 (CONTROL). Inclusion criteria: Non-diabetic children aged 8-12, attending a state health care center. Exclusion criteria: Children with other systemic conditions, with orthodontic treatment, who do not sign the consent form, receiving health care from a private provider.
Among the diabetic subjects, we measured the time elapsed between the onset (first diagnosis), which resulted in 8 children of up to 2 years of age, 11 children aged 2-5, and 10 children over 5 years of age.
VARIABLES. The oral health variables under study are:
DATA COLLECTION. The clinical oral examination was systematized and conducted by a single operator, who had been previously calibrated: Kappa intraoperator = 0.7. Throughout the data collection process, he continued the calibration maintenance (5%). The data from both groups were stored in a database that was used exclusively for this study.
ETHICAL CONSIDERATIONS. The people responsible for the project undertake to keep the data confidential. Signature of the free, informed and express consent of the parents and adults in charge, and the consent of the children. All the children received their oral health diagnosis, health education, and relevant oral hygiene measures, all at no cost to them. They all received an oral hygiene kit. This study was conducted with institutional endorsement and the approval of the Ethics Committee of the School of Dentistry of Universidad de la República.
RESOURCES AND FUNDING. The material resources were provided by the School of Graduates of the School of Dentistry, by way of funds granted by the Postgraduate Academic Commission of UdelaR.
STATISTICAL ANALYSIS. Descriptive statistics were calculated for all variables and for both groups of subjects. We studied the distribution of the variables which are relevant to oral health in order to compare the differences between diabetic and healthy subjects. All the data were processed using the SPSS statistical software.
Results
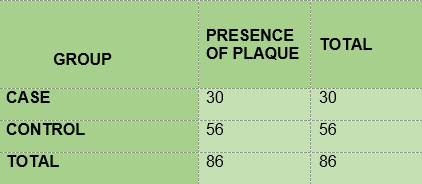
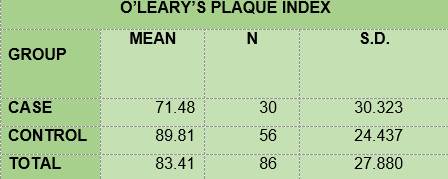
BIOFILM. All the children in group DM1 and in the control group had biofilm (Table 2). The mean for O’Leary’s modified visible plaque index (VPI) was lower among the diabetic children (71.48) than among the non-diabetic children (89.91) (Table 3).
DENTAL CARIES
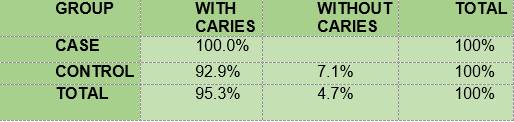
Presence of carious lesions according to the ICDAS II-Nyvad criterion. Table 4 shows the percentage of carious lesions in the case group and in the control group.
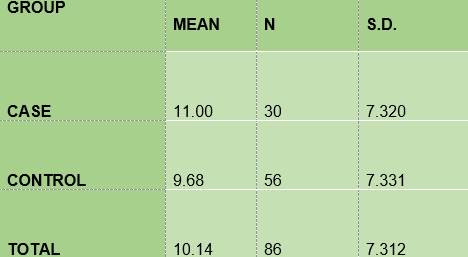
Caries surface according to the ICDAS II-Nyvad criterion - The mean of the number of surfaces with carious lesions among the diabetic subjects (11.00) is slightly higher than that of the non-diabetic subjects (9.68), (Table 5).
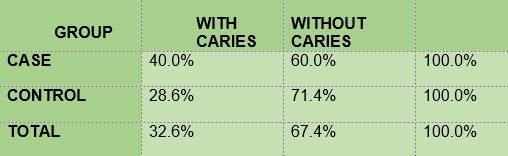
Caries according to the CPO criterion. The percentage of children with carious lesions among the diabetic subjects (40) was slightly higher than the percentage of non-diabetics (28.6) (Table 6).
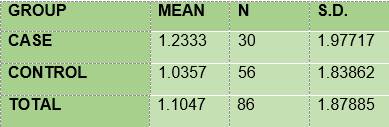
DMFT mean. The mean of DMFT among the diabetic subjects (1.233) was slightly higher than that of the non-diabetic subjects (1.035) (Table 7)
GINGIVAL INFLAMMATION
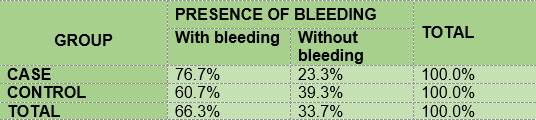
The percentage of children with gingival bleeding as measured by the GBI (J. Ainamo and I. Bay 1975) was slightly higher among the diabetic subjects (76.7) than among the non-diabetic subjects (60.7) (Table 8).
The mean for the bleeding index among the diabetic subjects (5.57) was higher than that of the non-diabetic subjects (2.36) (Table 9).
In summary, the results show that 100% of the members of the two groups under study had plaque; the mean O’Leary’s plaque index in the diabetic subjects is 71.48 while in the control group it was 89.81. Regarding gingival inflammation: 76.7% and 60.7% of the subjects had bleeding on probing, in the case group and the control group respectively, with a mean bleeding on probing index of 5.57 and 2.36, respectively. From the caries survey, conducted using the DMFT index, there arose that 40% of the members of the DM1 group had caries, while in the control group the figure was 28.6%; thus, the mean DMFT is slightly higher among the diabetic subjects (1.233) than in the control group (1.0357). The caries survey conducted according to ICDAS showed that 100% of the members of the case group had carious lesions, while in the control group the percentage was 92.9. Upon analysis of the number of surfaces with lesions, the mean in the case group was 11.0, slightly higher than the control group, 9.68.
Discussion
Based on the literature studied, oral health is affected in children who suffer from DM. The numerous studies that have researched the connection between dental caries and diabetes mellitus do not report unanimous findings. However, regarding gingival disease, most authors agree that there is a higher prevalence and severity among children with diabetes, and that it has an early onset 20. López del Valle’s (Puerto Rico, 2011) (21) case-control study conducted among children aged 6-12 (25 diabetic children in the case group and 25 healthy children in the control group) reported significant differences when comparing the data collected in the survey. It described higher levels in children suffering DM1: a higher VPI, the mean of the DM1 group was 2.5 and that of the control group was 0.8; a higher number of carious lesions in permanent teeth, 1.43 in the DM1 group and 0.56 in the control group; a higher bleeding on probing index, by area (GBI): 23.9% in the DM1 group and 4.2% in the control group. Lalla et al. (New York, 2007) 13) case-control study conducted among children and adolescents aged 6-18 (186 diabetic children in the case group and 160 healthy children in the control group) reported significant differences between the two groups. It reported higher values in children and adolescents suffering from DM1: a higher VPI, the mean of the DM1 group was 1.2 and that of the control group was 1.1, and a higher bleeding on probing index, by area (GBI): 23.6% in the DM1 group and 10.2% in the control group. However, it reported a lack of significance in the comparison of carious lesions between the groups.
The results of this study only showed statistically significant differences for the gingival bleeding index: the Mann-Whitney test showed that the bleeding index was significantly higher among the diabetic subjects (mean=3.65) than among the healthy ones (mean=1.04), U=517, p=0.03. Based on these analyses and on the results of this study, we agree with Novotna (2015) 12, whose revision article sets forth that the studies which associate DM and dental caries are inconclusive. In our case, the relation between DM and dental caries was not statistically significant. The analysis of the subjects’ diet was limited by the difficulty in counting the number of daily intakes as a risk factor for caries. In this respect, it was possible to obtain accurate data for group 1, given that they have a programmed feeding regimen which regulates the frequency and the quality of the intakes, but this was not possible in the control group, as most of the subjects eat food or drink juice in unlimited amounts and quite frequently between meals. The author also reported that most of the studies reviewed show a higher plaque and gingival inflammation index in the group of children and adolescents suffering from DM1, as compared to the control group of healthy subjects. DM has been proven to be a relevant risk factor for the development of periodontal disease 22-23 and a higher rate of prevalence and severity is seen in children suffering from diabetes, which has an early onset. Likewise, there is evidence that gingival inflammation may contribute to the persistence of hyperglycemia, thus causing poor glycemia control in people with DM 24-25). The mechanism in charge of the relationship between hyperglycemia and periodontitis, according to Taylor et al.26, is the development of a hyperinflammatory response to the bacterial challenge, which would broaden the range of changes in the diabetic host, including a defective barrier of the neutrophils which, together with an exaggerated response of the monocytes (increasing the liberation of proinflammatory cytokines and the oxidative reactions), would alter defense and healing mechanisms.
Conclusions
DM can have a significant impact on children’s oral health. It is therefore important for dentists and family to be familiar with the signs and symptoms which may be altered if some factors are not controlled. The results obtained in this study are the first data for Uruguay, and they provide elements to improve oral health protocols for children and adolescents, whether they suffer from DM1 or not. Likewise, the study has allowed us to analyze the results obtained, bearing in mind the results reported by international studies. Although the results validate the clinical relevance of this kind of study, its small sample size is a limitation, as it is understood that with small samples it is statistically less possible to detect significant differences than in larger samples. Health promotion through the encouragement of a healthy diet, good oral hygiene habits, and the metabolic control of diabetes must start at an early age, and the dentist must be part of the team treating diabetic children to help prevent complications. It is also paramount to include dental checkups for children who suffer from diabetes mellitus in the institutional protocols which lay down the guidelines for their comprehensive care, in order to contribute to and early diagnosis through an active role
Referencias
1. Sarwar N, Gao P, Seshasai SR, Gobin R, Kaptoge S, Di Angelantonio, Ingelsson E, Lawlor DA, Selvin E, Stampfer M, Stehouwer CD, Lewington S, Pennells L, Thompson A, Sattar N, White IR, Ray KK, Danesh J. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Emerging Risk Factors Collaboration. Lancet. 2010; 375 (9733): 2215-2222. [ Links ]
2. Rojas J, Bermúdez V, Leal E, Cano R, Luti Y, Acosta L, Finol F, Aparicio D, Arraiz N, Linares S, Rojas E, Canelón R, Sánchez D, Velasco M. Insulinorresistencia e hiperinsulinemia como factores de riesgo para enfermedad cardiovascular. Archivos Venezolanos de Farmacología y Terapéutica. 2008; 27 (1): 30-40. [ Links ]
3. Pettitt DJ, Talton J, Dabelea D, Divers J, Imperatore G, Lawrence JM, Liese AD, Linder B, Mayer-Davis EJ, Pihoker C, Saydah SH, Standiford DA, Hamman RF. Prevalence of diabetes in U.S. youth in 2009: the SEARCH for diabetes in youth study. Diabetes Care. 2014; 37 (2): 402-8. [ Links ]
4. DiaMond. Diabetes Mondiale Project Group. The DiaMond Project. Disponible en: http://www.pitt.edu/~iml1/diabetes/DIAMOND.html [ Links ]
5. Eurodiab. The Epidemiology and prevention of Diabetes. Int J Epidemiolog 1993;22. Disponible en: https://ec.europa.eu/research/success/en/med/0283e.html [ Links ]
6. International Diabetic Federation. Diabetes in the Young: A global Perspective. IDF diabetes Atlas Four Edition 2010. Disponible en: http://www.idf.org/sites/default/files/Diabetes%20in%20the%20Young_1.pdf [ Links ]
7. Ferrero R, García MV. Encuesta de prevalencia de la diabetes en Uruguay. Primera fase: Montevideo. Año 2004. Arch. Med. Int. 2005; 27 (1): 7-12. [ Links ]
8. American Diabetes Association. 2. Classification and diagnosis of diabetes: Standards of Medical Care in Diabetes. Diabetes Care 2018; 41 (Suppl. 1): S13-S27. [ Links ]
9. Miranda X. Caries e índice de higiene oral en niños con Diabetes Mellitus Tipo 1. Rev Chil Pediatr. 2013; 84 (5): 527-531. [ Links ]
10. Carcavilla Urquí A. Atención al paciente con diabetes: algo más que insulinas. Pediatr Aten Primaria. 2009; 11 (supl.16) [ Links ]
11. ISPAD. Clinical Practice Consensus Guidelines 2006-2007. Definition, epidemiology and classification. Pediatric Diabetes. 2006; 7: 343-51. [ Links ]
12. Novotna, Podzimek S, Broukal Z, Lencova E, Duskova J. Periodontal diseases and dental caries in children with type 1 Diabetes Mellitus. Mediators Inflamm. 2015; 2015: 379626. [ Links ]
13. Lalla E, Cheng B, Lal S, Kaplan S, Softness B, Greenberg E, Goland RS, LamsterIB. Diabetes mellitus promotes periodontal destruction in children. J ClinPeriodontol 2007; 34: 294-298. [ Links ]
14. Lalla E, Cheng B, Lal S, Tucker S, Greenberg E, Goland R, Lamster IB. Periodontal Changes in Children and Adolescents with Diabetes. A case-control study. Diabetes Care. 2006; 29 (2): 295-299. [ Links ]
15. Hamman R, Bell R, Dabelea D, D'Agostino R.Jr, Dolan L, Imperatore G, Lawrence JM, Linder B, Marcovina S, Mayer-Davis EJ, Pihoker C, Rodriguez B, Saydah S. The Search for diabetes in youth study: Rationale, Findings and Future Directions. Diabetes Care. 2014; 37 (12): 3336-3344. [ Links ]
16. Szwarc E., López Jordi M.C. Salud bucal en niños y adolescentes portadores de diabetes tipo 1. Conference Paper: June 2015. IX CLIOA, ISBN 978-858842513-2. Disponible en: https://www.researchgate.net/publication/278727314_Salud_Bucal_En_Ninos_Y_Adolescentes_Portadores_De_Diabetes_Mellitus_Tipo_1 [ Links ]
17. Cuenca Sala E, Manau Navarro, C, Serra Majem L. Odontología Preventiva y Comunitaria. 2ª ed. Barcelona: Masson SA; 2001. [ Links ]
18. Ismail AI, Sohn W, Tellez M, Amaya A, Sen A, Hasson H, Pitts NB. International Caries Detection and Assessment System (ICDAS): an integrated system for measuring dental caries. Community Dent Oral Epidemiol. 2007; 35 (3): 170-8. [ Links ]
19. Ainamo J, Bay I. Problems and proposals for recording gingivitis and plaque. Int Dent J. 1975; 25 (4): 229-35. [ Links ]
20. Palomer L, García H. ¿Es importante la salud oral en los niños con diabetes? Rev Chil Pediatr 2010; 81 (1): 64-70 [ Links ]
21. López del Valle LPR. Comparing the Oral Health Status of Diabetic and Non-Diabetic children from Puerto Rico: a Case-Control Pilot Study, PR Health Sci J. 2011 Sep; 30 (3):123-127. [ Links ]
22. Mealey BL, Ocampo GL. Diabetes mellitus and periodontal disease. Periodontol. 2000. 2017; 44: 127-153 [ Links ]
23. Duran-Merino D, Molina-Frechero N, Castañeda-Castaneira E, Gaona E, Reyes-Reyes RE, Tremillo-Maldonado O, del Muro-Delgado R, Juárez-López ML, Bologna-Molina R. Relationship between periodontal disease and Type 1 Diabetes in adolescents. Ann Med Health Sci Res. 2017; 7: 350-354. [ Links ]
24. Winning L, Patterson CC, Neville CE, Kee F, Linden GJ. Periodontitis and incident type 2 diabetes: a prospective cohort study. J Clin Periodontol. 2017; 44:266-274. [ Links ]
25. Albandar JM, Susin C, Hughes FJ. Manifestations of systemic disease and conditions that affect the periodontal attachment apparatus: Case definitions and diagnostic considerations. J Periodontol. 2018; 89 (Suppl 1): S183-S203. [ Links ]
26. Taylor JJ. PreshawPM, LallaE. A review of the evidence for pathogenic mechanisms that may link periodontitis and diabetes. J Periodontol. 2013; 84 (4 Suppl.): S113-S114. [ Links ]
Received: July 02, 2018; Accepted: August 02, 2018











 text in
text in