Servicios Personalizados
Revista
Articulo
Links relacionados
Compartir
Odontoestomatología
versión impresa ISSN 0797-0374versión On-line ISSN 1688-9339
Odontoestomatología vol.19 no.spe Montevideo set. 2017
https://doi.org/10.22592/ode2017n.esp.p68
Articles
In vitro study of measures to control microbial colonization in the internal chamber of the implant and the implant-abutment system
1Cátedra Rehabilitación Prostodoncia Fija y TTM. Director Departamento Implantología Oral y Maxilofacial, Director de Carreras de Especialización en Implantología Oral y en Prostodoncia. Facultad de Odontología. Universidad de la República. Uruguay. pablojuliopebe@gmail.com
2Cátedra Rehabilitación Prostodoncia Fija y TTM. Integrante del Departamento de Implantología Oral. Docente Carrera de Especialización en Implantología Oral. Facultad de Odontología. Universidad de la República. Uruguay.
3Cátedra de Microbiología General y Bucodental, Directora Carreras de Asistente e Higienista en Odontología. Facultad de Odontología. Universidad de la República. Uruguay.
Abstract. Objective: To determine biofilm control measures in the internal chamber of the implant through an in vitro test. Method: Different antimicrobial agents were selected and placed in the chambers of three groups of implants. After seven days, we immersed them in a microbial suspension for incubation. We collected, cultured and incubated the samples from the chambers of each study group. Results: We detected a filtration of microorganisms into the internal chamber in all the groups of implants studied. This resulted in a higher count of colony-forming units (CFU) in the control group, whereas in the experimental groups we identified a significant reduction in the CFU count. Conclusions: We observed a significant decrease in the number of CFU in the experimental groups in relation to the control group, which determines the advantage of using this type of antimicrobials
Keywords: microbial flora; dental implants; internal chamber; infection control
Resumen: Objetivo: Determinar mediante un ensayo in vitro, medidas de control del biofilm en la cámara interna del implante. Método: Se seleccionaron diferentes agentes antimicrobianos que se colocaron en las cámaras de 3 grupos de implantes. Luego de 7 días de inmersos en una suspensión microbiana e incubados, realizamos la toma, cultivo e incubación de las muestras de las cámaras de cada grupo de estudio. Resultados: Se constató una filtración de microorganismos hacia la cámara interna en todos los grupos de implantes estudiados, obteniéndose un mayor recuento de Unidades Formadoras de Colonias (UFC) en el grupo control, mientras que en los grupos experimentales se identificó una reducción significativa en el recuento de UFC. Conclusiones: Se observó una disminución significativa en la cantidad de UFC en los grupos experimentales respecto al grupo control, lo que determina la ventaja de utilizar este tipo de antimicrobianos
Palabras clave: Flora microbiana; implantes dentales; cámara interna; control de la infección
Introduction
The implant practice has made critical contributions to the functional and aesthetic rehabilitation of full and partial edentulism. Very successful treatments have been designed and protocolized in each situation with high implant and prosthesis survival and success rates, whatever the type and modality of treatment1. However, it has been shown that microbial colonization in the implant-abutment-restoration system with a prevalence of Porphyromonas gingivalis, Prevotella intermedia and Aggregatibacter actinomycetemcomitans, among others, can cause peri-implant disease, characterized as mucositis in the marginal inflammation stage, and as peri-implantitis when the bone-implant interface is invaded and colonized by bacterial activity causing a peri-implant infection1. This can occur from the time when the implant is placed, attributed to the flora present in the oral cavity, and also during the second-stage surgery, when it is attributed to the infiltration in the gap between the abutment and the implant, and between the abutment and the restoration, once the prosthesis has been placed. This gap is generally found at the level of the alveolar crest, is susceptible to bacterial colonization and represents a reservoir of microorganisms2,3. .Different preventive and therapeutic measures aimed at controlling biofilm in the area surrounding the implant-supported restoration have been proposed to deal with this widely studied phenomenon3. Another critical area, the internal chamber of the implant and the implant-abutment-restoration system, is added to this process. Contamination of these sites during second-stage surgery or the execution of prosthetic procedures, or even during the placement of the final prosthesis, results in colonization inside a closed environment that is inaccessible to oral hygiene products (mechanical and chemical)4. In a study conducted 14 days after placing the prosthesis, the count of microorganisms was higher in cast abutments versus machined abutments, which confirms microfiltration in the gap between the abutment and the implant5. This exchange goes both ways: there is bacteria entering the chamber and coming out of it2,6. Hence, the internal chamber and the abutment-implant connection represent a reservoir of microbial activity that can affect the area surrounding the implant7. .Internal contamination is frequent and can persist for long periods of time. Microbial leakage is observed in all the implant and abutment systems analyzed8,11.
Rationale
Traditional protocols have not solved the problem presented above and, consequently, no specific measures have been developed to control internal colonization. To reduce microleakage, abutments are developed using nitinol (shape memory metal), which in in vitro studies reduces the microgap to 1 micron. Another path tested is using antimicrobial agents. Tests have been conducted placing chlorhexidine inside implants, which raises the possibility of reducing the microbial population at that level1,13. Groenendijk et al.14 tested the effects of 2% chlorhexidine and obtained an initial promising result with a significant CFU reduction (48% in the control-saline group vs. 17% in the test-2% chlorhexidine group). Periodontal indicators, such as gingival fluid flow, plaque index and gingival index, do not vary significantly in neither group. There is a positive effect on the peri-implant tissue, since chlorhexidine can permeate from the inside through the micrograp and reduce colonization in the gingival sulcus. The long-term effects of this procedure are questioned because of the short half-life of the product once it has been applied. No conclusive results are observed in the study conducted by Groenendijk E. et al. regarding the effective control of the internal microbial contamination of the implant using chlorhexidine14. Oral microflora plays an important role in the beginning and perpetuation of the infectious process in the oral cavity. This is why investigating and identifying it contributes to the selection and application of preventive measures, defining the etiology of the infection and applying the appropriate treatment15. The application of molecular methods to identify oral pathogens makes it possible to better manage and follow-up patients, an example of which is multiplex-PCR, which enables the simultaneous detection of colonizing microorganisms in the internal chamber of the implant and in the implant-abutment system16.
The general objective of this in vitro study was to develop different biofilm control measures in the internal chamber of the implant by detecting the bacterial species present, as well as the possible effect of antimicrobial agents used on implant surfaces. The specific objectives were: 1) to determine the action of different antimicrobial agents (calcium hydroxide, iodoform and combinations) against the biofilm flora, 2) to quantify the microbiota, 3) to draw conclusions for clinical application in the control of the microbial colonization of the internal chamber of the implant and the implant-abutment system.
Materials and methods
This is described as an experimental, in vitro study.
1. Selection of antimicrobial measures - The following criteria were considered when selecting antimicrobial agents:
Is specific regarding the prevalent flora
Does not affect the integrity of the components of the implant-abutment-restoration system
Does not act in any case as a cementing material
Is economical, has a pleasant taste and is easily handled
Does not attack gingival tissues.
The antimicrobial agents selected were: iodoform, calcium hydroxide and a 50/50 combination of both (calcium hydroxide powder, Leduc®, iodoform powder, Leduc ®). Methylcellulose (Leduc ®) was used as medium in all cases.
2. Bacterial strains- The following strains were used:Porphyromonas gingivalis (BAA-308), Prevotella intermedia (ATCC 25611), Tannerella forsythia (ATCC 43037) and Fusobacterium nucleatum (ATCC 25586).
3. Study groups:
1.1. We used 80 cylindrical titanium implants with external connections measuring 8.5 mm in length and 3.75 in diameter (3i FI-USA®), with their corresponding cover screws, and formed four groups:
a) Control group (CG) : 20 implants with nothing placed inside the internal chamber of the implant.
b) Experimental group 1 (EG1):20 implants with the internal chamber of the implant coated with calcium hydroxide and methylcellulose.
c) Experimental group 2 (EG2):20 implants with the internal chamber of the implant coated with iodoform and methylcellulose.
d) Experimental group 3 (EG3): 20 implants with the internal chamber of the implant coated with calcium hydroxide, iodoform and methylcellulose.
Disposable sterile needles and syringes were used to introduce the chemical agents, and sterile No. 25 paper points were used to collect samples. The implants of the CG were placed in a sterile bottle with thioglycolate17,18 and a suspension of bacteria was introduced in the culture medium.
In the experimental group 1 (EG1), consisting of 20 implants, we applied calcium hydroxide in the corresponding chambers (Fig.1). To that end, 1 g of this compound was weighed on an electronic scale using an aseptic technique. It was poured on sterile glass, two drops of methylcellulose were dropped on it, and then it was combined. We opened an implant, removed the cover and, using a disposable needle and syringe, took the suspension and introduced a drop into the chamber.
We proceeded to place the cover screw and wipe off the excess using sterile gauze. Once clean, we placed the implant in a sterile bottle with the thioglycolate medium and the bacteria suspension using sterile pliers.
In the experimental group 2 (EG2), we applied iodoform with methylcellulose in the corresponding chambers. To that end, 1g of this compound was weighed on an electronic scale using an aseptic technique. It was poured on sterile glass, two drops of methylcellulose were dropped on it, and then it was combined. We opened an implant, removed the cover screw and, using a disposable needle and syringe, took the suspension and introduced a drop of it into the chamber. We proceeded to place the cover screw and wipe off the excess using sterile gauze. Once clean, we placed the implant in the culture in a thioglycolate medium using sterile pliers. The same procedure was carried out with the remaining 19 implants.
In the experimental group 3 (EG3), we placed calcium hydroxide and iodoform with methylcellulose in the corresponding chambers. To that end, 1 g of calcium hydroxide and 1 g of iodoform were weighed on an electronic scale applying an aseptic technique. We poured it on sterile glass, dropped two drops of methylcellulose on it, and then combined the mixture. We opened an implant, removed the cover screw and, using a disposable needle and syringe, took a sample from the suspension and introduced a drop of it into the chamber. We then placed the cover screw and wiped off all the excess material with sterile gauze. Once clean, the implant was placed in the culture with the thioglycolate medium using sterile pliers. The procedure was repeated with the remaining 19 implants. The bottle contained a thioglycolate culture medium with a suspension containing 107-8x108 CFU of Porphyromonas gingivalis, Prevotella intermedia, Tannerellaforsythia and Fusobacterium nucleatum. These bacteria are among the most prevalent in peri-implant disease17,21) (Fig. 2).
Bottles were incubated at 37°C for 7 days (Fig. 3). After that time had elapsed, the implants were removed from the thioglycolate culture using sterile pliers, and then they were cleaned and dried with sterile gauze. After removing the cover screws, sterile No. 25 paper points were introduced (Fig. 4) inside each internal chamber of each of the implants of the four groups. Each of the 80 samples was introduced in one of 80 tubes containing a solution of 1.5 ml of RTF (Reduced Trasport Fluid)22. All implant handling (screwing and unscrewing of cover screws) was performed by the same operator.
Dilutions were then prepared. One rack was used per sample with: one eppendorf tube with 1.5 ml of RTF with the corresponding two No. 25 paper points and two eppendorf tubes with 900 μl of RTF. Then the sample was diluted for quantification (Fig. 5). In each sample there was 1.5 ml of RTF with the corresponding two No. 25 paper points. 100 µl was taken from the sample and placed in an eppendorf tube with 900 µl of RTF. (First dilution: 1:10). Then, 100 µl was taken from the first dilution and placed in another tube with 900 µl of RTF (second dilution: 1:100). 100 µl was taken from the first and 100 µl from the second and streaked in base agar medium with blood, menadione and hemin, expanded with a glass spreader, and the plates were incubated for 14 days at 37ºC in absolute anaerobiosis23-24.
After the incubation period, the plates were read by performing the CFU (colony-forming units) count for each study group, using a stereoscopic magnifying glass.
Results
Plates were opened after 14 days. (Figs. 6 and 7)
Control group- Samples obtained from the implant chambers without chemical compounds. All of the plates streaked with the two dilutions showed colony growth. The samples streaked from the first dilution showed: 2 plates with more than 3,000 CFU, 2 plates with 6 CFU, 8 plates with 4 CFU, 6 plates with 3 CFU, 2 with 1 CFU. The samples streaked from the second dilution showed:2 plates with more than 100 CFU, 2 plates with 5 CFU, 2 plates with 3 CFU, 2 plates with 4 CFU, 6 with 2 CFU, 4 with 1 CFU and 2 plates without growth.
EG1- Samples obtained from the implant chambers with calcium hydroxide and methylcellulose. Of the plates streaked with the first dilution, one showed 2 CFU, second dilutions did not show growth of microorganisms.
EG2- Samples obtained from the implant chambers with iodoform and methylcellulose. Two of the plates streaked from the first dilution of the samples showed growth with 2 CFU. Two of the plates streaked from the second dilution of the samples showed growth with 1 CFU.
EG3- Samples obtained from the implant chambers with the mixture of calcium hydroxide, iodoform and methylcellulose. Two of the plates streaked from the first dilution showed growth with 1 CFU. The samples streaked from the second dilution showed no growth.
The results of this study show that there was a leakage of microorganisms into the internal chamber of the implant. There was colony growth in all the plates streaked with the samples without chemical agents (100%). The use of the chemical agents introduced in the chambers of implants was favorable because they reduced the number of colony-forming units in all samples, both in the first dilutions (95% in EG1 and 90% in EG2 and EG3) and in the second dilutions (90% in EG2 and 100% in EG1 and EG3), with the most successful result being those obtained using the mixture of calcium hydroxide and methylcellulose, and the combination with iodoform and methylcellulose.
The descriptive analysis of the results identified a substantial difference in CFU growth in the group “without agent” (control group) in relation to the other experimental groups, with a reduction in the second dilution, but no significant reduction in the CFU count was found between them (Fig. 8). The use of calcium hydroxide, whether alone or in combination with iodoform, showed the lowest CFU count among the groups studied. It was not possible to determine the duration of the effect of this agent.
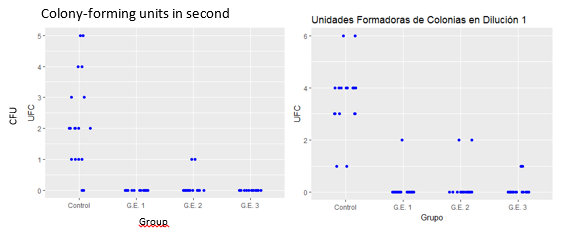
Fig. 8: The average CFU in the control group was 3.56, in the experimental group 1, 0.1, in the GE2,0.2 and in the GE3,0.1. A significant decrease in the number of CFU in the groups treated with some antimicrobial agent in relation to the control group was observed. No significant difference was observed when comparing the different experimental groups. The same results were obtained when analyzing the results of the second dilution.
Discussion
This study enabled the comparison of two antimicrobial agents, and a combination of both, in relation to the microflora which colonizes the internal chamber of the implant. We could determine which antimicrobial agent was more effective against the flora in the internal chamber of the implant. Works which study this phenomenon of contamination in the implant-abutment-restoration system1,2,4,7,12-14,21 use chlorhexidine as an antimicrobial agent with varying results and note its short life as a common characteristic. The selection of calcium hydroxide and iodoform appears as an alternative to chlorhexidine in trying to achieve a higher effectiveness against contamination. No studies using the antimicrobials used in this work were found in the review of the literature conducted. These are the antimicrobials used in the daily dentistry practice with the same concentration, which guarantees product biocompatibility. After reading the cultures, it was possible to establish, with samples from 20 implants each, that calcium hydroxide alone or in combination with iodoform showed the best performance, since the growth of colony-forming units observed in the cultures in which this product was used was minimal, although we cannot determine a significant difference with the group where only iodoform was used as an antimicrobial (GE2).
Bacterial strains which can regularly colonize these completely anaerobic zones were used in this research. It is important to conduct an in vivo study to assess the mid and long-term effectiveness of these measures.
Conclusions
We found a significant decrease in the growth of CFU in the experimental groups in relation to the control group, which determines the advantage of using calcium hydroxide and iodoform as antimicrobial agents to control the growth of microorganisms inside the internal chamber of dental implants. These results open the door for further research on the fight against contamination in the implant chamber, whether by expanding the sample in in vitro studies or by conducting a prospective clinical study (upcoming) which will make it possible to confirm the results obtained in the laboratory
Referencias
1.Gotfredsen K, Berglundh T, Lindhe J. Anchorage of titanium implant with different surface characteristics: an experimental study in rabbits. Clin Implant Dent Relat. Res. 2000; 2(3): 120-128 [ Links ]
2.Quirynen M, van Steenberghe D. Bacterial colonization of the internal part of two-stage implants. An in vivo study. Clin Oral Implant Res. 1993; 4: 158-161. [ Links ]
3.Broggini N, McManus LM, Herman JS, Medina RU, Oates TW, Schenk RK, Buser D, Mellonig JT, Cochran DL. Persistent Acute Inflammation at the Implant-Abutment Interface. J Dent Res. 2003; 82(3): 232-237. [ Links ]
4.Besimo C, Guindy J, Lewetag D, Meyer J. Prevention of bacterial leakage into and from prefabricated screw-retained crowns on implants in vitro. Int J Oral Maxillofac Implants. 1999; 14:654-660. [ Links ]
5.Persson L G, Lekholm U, Leonhardt A, Dahlén G, Lindhe J. Bacterial colonization on internal surfaces of Branemark system implant components. Clin Oral Impl Res. 1996: 7:90-95. [ Links ]
6.Do Nascimento C, Barboza RE, Issa JPM, Watanabe E, Ito IY, Albuquerque Jr RF. Bacterial leakage along the implant-abutment interface of premachined or cast components. Int J Oral Maxillofac Implants. 2008; 37: 177-180 [ Links ]
7.Quirynen M, Bollen CML, Eyssen H, van Steenberghe D. Microbial penetration along the implant components of the Branemark system, an in vitro study. Clin Oral Implant Res. 1994; 5: 239-244. [ Links ]
8.Assenza B, Tripoldi D, Scarano A, Perrotti V, Piatelli A, Iezzi G, D´Ercole S. Bacterial Leakage in Implants with Different Implant-Abutment Connections: An In Vitro Study. J.Periodontol. 2012;83:491-497. [ Links ]
11.Steinebrunner L, Wolfart S, Bobmann K, Kern M, In Vitro Evaluation of bacterial leakage along the Implant-Abutment interface of different Implant systems. Int J Oral Maxillofac Implants 2005;20:875-881. [ Links ]
12.Cortizo MC, Oberti TG, Cortizo MS, Cortizo AM, Fernandez Lorenzo de Mele M. Chlorhexidine delivery system from titanium/polybenzyl acrylate coating: Evaluation of cytotoxicity and early bacterial adhesion. J Dent. 2012; 40: 329-337 [ Links ]
13.Quirynen M, De Soete M, van Steenberghe D. Infectious risks for oral implants: a review of the literature. Clin Oral Impl. Res. 2002; 13: 1-19. [ Links ]
14.Groenendijk E, Dominicus J, Moorer W, Van Waas M. Microbiological and clinical effects of clorhexidine enclosed in fixtures of 3i-Titamed implants. Clin Oral Impl Res. 2004; 15: 175-179 [ Links ]
15.Santos Barboza RE, do Nascimento C, Mardegan JP, Watanabe E, Yoko I, de Albuquerque RF, Bacterial cultura and DNA checkerboard for the detection of internal contamination in dental implants. J Prosthodon. 2009; 18:376-381. [ Links ]
16.Koyanagi T, Sakamoto M, Takeuchi Y. Analysis of microbiota associated with peri-implantitis using 16 S rRNA gene clone library. J Oral Microbiol. 2010; 24(2). Doi: 10.3402/jom.v2i0.5104. [ Links ]
17.Bürgers R, Gerlach T, Hahnel S, Schawartz F, Handel G. In vivo and in vitro biofilm formation on two different titanium implant surfaces. Clin Oral Implants Res. 2010; 21(2):156-64.Epub 2009,Nov,13. [ Links ]
18.Duarte PM, Reis AF, de Freitas PM, Ota-Tsukuki C. Bacterial adhesion on smooth and rough titanium surfaces after treatment with different instruments. J.Periodontol. 2009; 80(11):1824-32. [ Links ]
21.Kern M, Harder S, Antimicrobial filling of implant cavities. J Prosthet Dent. 2010;103: 321-322. [ Links ]
22.Syed SA, Loesche WJ. Survival of human dental plaque flora in various transport media. Applied Microbiolology 1972; 24(4): 638-644 [ Links ]
23.Gajardo M, Silva N, Gómez L, León R, Parra B, Contreras A, Gamonal J. Prevalence of Periodontopathic: Actinobacillus actinomycetemcomitans Bacteria in Aggressive Periodontitis Patients in a Chilean Population. J Periodontol. 2005; 76: 289-294. [ Links ]
24.Herrera D, Contreras A, Gamonal J, Oteo A, Jaramillo A, Silva N, Sanz M, Botero J, León R. Subgingival microbial profiles in chronic periodontitis patients from Chile, Colombia and Spain. J Clin Periodontol. 2008;35: 106-113 [ Links ]
Received: March 23, 2017; Accepted: July 06, 2017











 texto en
texto en 









