Serviços Personalizados
Journal
Artigo
Links relacionados
Compartilhar
Odontoestomatología
versão impressa ISSN 0797-0374versão On-line ISSN 1688-9339
Odontoestomatología vol.19 no.spe Montevideo set. 2017
https://doi.org/10.22592/ode2017n.esp.p46
Articles
Workshop 5 - Peri-implantitis
1Cátedra de Rehabilitación, Prostodoncia Fija y TTM. Facultad de Odontología. Universidad de la República. Uruguay. dsales@montevideo.com.uy
2Cátedra de Periodoncia. Facultad de Odontología. Universidad de la República. Uruguay.
3Cátedra de Rehabilitación, Prostodoncia Fija y TTM. Departamento de Implantología Oral y Maxilofacial. Facultad de Odontología. Universidad de la República. Uruguay.
Working methodology at the peri-implantitis workshop. The workshop was divided into three stages: 1) development of guiding topics, selection and delivery of articles, 2) reception, evaluation and returning of articles, and 3) the workshop itself.
FIRST STAGE: The process began in February 2014, when five guiding questions were formulated to organize the workshop discussion. These were: Diagnosis, Epidemiology, Risk Factors, Periodontal Disease History and Peri-Implantitis Treatment. A literature search was performed in PUBMED, EMBASE, LILACS and COCHRANE, from 2000 to date, in both English and Portuguese, using the following descriptors: peri-implantitis, peri-implant disease, perimplantitis, risk factor, epidemiology, incidence, prevalence, treatment, therapeutics, combined with boolean operators. Most articles were cross-sectional, retrospective studies, systematic reviews, consensus statements, with few meta-analyses and prospective studies. Case reports and author reviews were excluded. Workshop leaders selected the most relevant articles, excluding consensus statements, and emailed the papers selected to the workshop participants.
SECOND STAGE: workshop participants received and analyzed the articles selected. The articles that met the inclusion criteria were distributed among the workshop participants. The workshop took place from 8:30 to 12:30hs, and 45 minutes were devoted to each guiding question. After the debate, the reviewer summarized and analyzed the selected articles, stating if the conclusions drawn were consistent with the evidence resulting from the literature considered.
Workshop leaders: Drs. Judith Esquenazi and Diego Sales. Secretary: Dr. Martín Minvielle. Reviewer: Dr. Ernesto Andrade
Workshop participants: Drs. Adriana Ramos, Carolina Verolo, Jorge Cabrera, Luis Arroyo, Magdalena Mayol, María José Quintana, Ricardo Kaufmann, Verónica Foglino, Virginia Papone, Michel Bittencourt, Carolina Aldaya, Alessia Molinari, Sebastián Pérez.
Question No. 2.- How to diagnose: Mucositis and Peri-implantitis? The term “peri-implantitis” was introduced by Mombelli in 19871, and then modified in the 1st European Workshop on Periodontology, to describe an inflammatory disease that leads to bone loss around dental implants2. Peri-implant diseases include: mucositis, described as an inflammatory lesion in the mucosa surrounding the implant, without concomitant bone loss3. The literature presents discrepancies regarding the clinical parameters to consider (Figure 1).
The connective tissue adjacent to the implant has abundant collagen fibers, is relatively acellular and avascular, and is histologically similar to scar tissue. Collagen fibers are arranged parallel to the axis of the implant, resulting in a more labile connection and faster disease progression for peri-implantitis compared to periodontal disease4.
Classification of peri-implantitis5
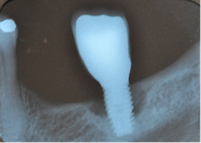
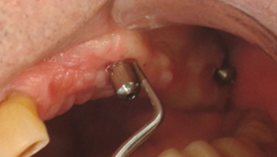
Early: Probing depth >4 mm, with bleeding and/or suppuration in >2 implant sites, and bone loss <25% of the total implant length (Fig. 2)
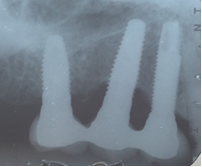
Moderate: Probing depth >6 mm, with bleeding and/or suppuration in >2 implant sites, and bone loss between 25% and 50% of the total implant length (Fig. 3)
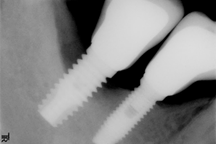
Advanced: Probing depth >8 mm, with bleeding and/or suppuration in >2 implant sites, and bone loss >50% of the total implant length (Fig. 4)
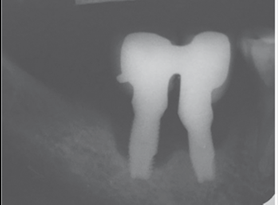
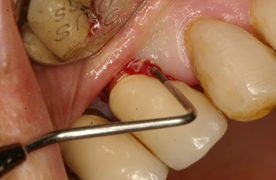
As in any pathology, the right diagnosis is essential to find a suitable treatment. The parameters to consider are: Bleeding on probing and/or Suppuration (Figure 5) and increased Probing depth (Figure 6) compared to initial exam and radiographic control. Other diagnostic methods provide little information, are more costly and complex to apply.
The presence of bleeding on probing and/or suppuration with a pressure lower than 0.25 N is a very useful parameter to diagnose mucositis and peri-implantitis. Numerous studies have found a direct link between bleeding on probing and suppuration and attachment loss around the implant. A prospective study that monitored the conditions of the peri-implant mucosa during supportive periodontal therapy found that bleeding on the same site, in over half of recall visits, in a period of two years, was related to the progression of the disease6.
Several studies have shown that an increase in probing depth entails an attachment and bone loss1,7,8,9. There is agreement that the pressure must not exceed 0.25 N, because this allows for full tissue recovery without consequences after five days10, while higher pressures can penetrate the tissues and reach the proximity of the bone crest. EUROPERIO 6o (2008) emphasized that emergence profile and contour of the reconstruction may make probing around the perimeter difficult, Therefore, it is suggested that at least one surface (mesial, distal, vestibular or lingual) should be explored11. In 2013, Serino et al.12 conducted a clinical study on 119 implants. They found that only 37% of the sites examined showed the same probing depth with and without the prosthesis in place. However, in 39% of the cases, the difference was 1 mm, and in 15% of cases it was 2 mm, which shows how difficult it is to conduct probing correctly after prosthesis placement. Additionally, in 66% of the implants, bone loss was similar in all four surfaces, while in the remaining cases it was not uniform, the vestibular surface being most affected4.
Radiographic examination. All authors agree on the importance of radiographic examination of implants over time. The main disagreement was on when the initial x-ray should be taken: when the implant is placed, when the restoration is placed or after bone remodeling. This workshop found greater scientific evidence, as well as agreement among authors, supporting initial radiographic examination, after post-restoration bone remodeling11,13,14.
Question No. 2. Epidemiology: what is the current prevalence? Epidemiology is the study of the distribution of the various diseases in populations. It draws on measurements such as prevalence and incidence, while attempting to elucidate the etiology and risk factors involved in the health-disease process. Zitzman & Berglundh concluded that the prevalence of peri-implantitis ranges between 28% and 56% of individuals (12%-40% of implants). Furthermore, peri-implant mucositis appears in approximately 80% of the subjects and 50% of the implants3,11. A recent meta-analysis of nine articles published until 2012 with 6283 implants in 1497 patients showed that 9.6% of implants present peri-implantitis. Considering the individual as the experimental unit, the number reaches 18.8%15. Up to 2002, only 40-60% of retrieved articles provided data on new cases of peri-implantitis. In turn, the lack of homogeneity in the clinical and para-clinical parameters used for recording the disease has made it impossible to draw conclusive results on the incidence of the pathology16. The epidemiological study of Peri-implant Disease (mucositis and peri-implantitis) must consider the following aspects:
most studies used samples that were convenient for the researcher (which may have been biased) and not selected by chance (randomly), which makes it impossible to generalize the results to the population (external validity)45;
there is no initial data (e.g., x-rays) to assess bone loss and estimate its evolution;
from an epidemiological standpoint, it is inappropriate to extract data on peri-implantitis prevalence only from one element or variable (e.g., bleeding on probing)46;
lack of unanimity on the definition of peri-implantitis and on the threshold of each variable used (probing depth and bleeding), which makes comparing studies harder to do23,46;
the inclusion of smokers and people with periodontitis modifies the disease prevalence, and these sub-populations are not always properly recorded and considered in the studies;
the use of the implant instead of the subject to quantify the disease (experimental unit of analysis), does not fully represent the frequency of the disease because it can underestimate the existing pathology;
Most studies published to date record cases of peri-implantitis five years after implant loading; however, some authors consider this amount of time insufficient for developing the disease, ten years being a better parameter16.
There are gaps in the information presented in the articles published, as there are unreported data and information47.
Question No. 3. Which are the risk factors? To identify a risk factor it is necessary to conduct true prospective studies to follow up on a specific population, and to establish a temporal connection between the exposure factor and the disease. There have been very few studies with these characteristics, and the available data generally stem from cross-sectional studies and series of cases that allow us to identify risk indicators17,18.
Which general, local and individual conditions can favor the appearance of peri-implantitis? The workshop concluded that risk indicators with greater evidence are:
Smoking: a meta-analysis that compares smokers and non-smokers identifies smoking as an indicator of significant risk of marginal bone loss, compared to non-smokers. This should be considered in the information provided to the patient before the treatment, as well as in the maintenance stage, where it is possible to detect early negative changes in peri-implant tissues17,19. Smoking has long-term deleterious effects on the immune and inflammatory response, as well as on healing, with a lower production of collagen, dysfunction of fibroblasts, reduction in peripheral circulation and in the function of neutrophils and macrophages20.
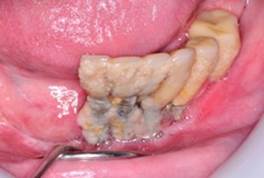
Poor oral hygiene: The accumulation of dental biofilm, caused by poor oral hygiene, leads to peri-implant disease11. The association between the amount of biofilm and peri-implantitis appears to be dose-dependent, as subjects with high values of biofilm are more likely to have a worse peri-implant condition21) (Fig.7).
History of periodontal disease (analyzed below as it was considered as a separate question).
On the other hand, lower-risk indicators are:
Diabetes: associated with xerostomia, dental caries and periodontal disease. Increased susceptibility to periodontal disease occurs due to the negative influence of hyperglycemia in the host’s inflammatory and immune mechanisms, resulting in a deregulated response, altered healing and microvasculature problems. It is not possible, for instance, to conduct a meta-analysis because of the heterogeneity of the data available22. A cross-sectional study of 212 subjects, with 578 implants, included 29 diabetics. The results of this study show that inadequate metabolic control in diabetic patients increases the risk of peri-implantitis. Peri-implantitis was diagnosed in 24.13% of diabetic patients, whereas in non-diabetics it was significantly less than 6.56%21.
Occlusal overload: The reports are controversial, as there is no unanimity in the definition of occlusal overload. Implants are considered more labile than teeth when facing axial forces given their lack of periodontal ligament23. Excessive stress can cause micro fractures and bone loss. A recent systematic review24 suggests that occlusal overload is positively associated with marginal peri-implant bone resorption. However, biofilm remains the key causal factor. More research is needed to define, with precision, what occlusal loading is23.
Alcoholism: A prospective three-year study evaluated the possible connection between marginal peri-implant bone loss and tobacco and alcohol consumption. The multivariate analysis showed that bone loss was significantly associated with the consumption of >10g of alcohol per day25.
Residual cement: a prospective study included 39 patients with 42 implants, with peri-implantitis, which had been restored with single cemented crowns. Twelve of them also had 20 restored implants under the same circumstances, but without peri-implantitis (this second group of implants was used as control group). A dental endoscope was used to explore the subgingival area (DentalView, Irvine, CA). As a result, residual cement was found in 34 of the 42 implants, and in none of the control implants. The cement was removed using different techniques (surgical and non-surgical). After 30 days, the signs of peri-implant disease had disappeared in 25 of the 33 implants placed in the experimental group. Therefore, excess cement and its incomplete removal from the subgingival surface of the implant provide an environment susceptible to bacterial colonization and the development of an inflammatory response26.
Surface of the implant: at present there is no evidence that the surface characteristics of the implant may have a significant influence on the onset the peri-implantitis27.
Absence of keratinized gingiva: The deficiency of keratinized mucosa around the implants seems to be related to clinical parameters of inflammation and biofilm accumulation. However, based on the evidence currently available, the amount of keratinized mucosa related to the development of the peri-implantitis remains controversial(28.29).
Question No. 4. Periodontal and peri-implant disease history. In the last few years, there has been an increase in the number of studies that analyze the relationship between implantology and periodontics. Implantology, created for the rehabilitation of fully edentulous patients, was later used to treat partially edentulous patients. Late implant failure seems to be strongly connected to the type of peri-implant bacterial flora and the conditions of the host30. The biofilm associated with implants with peri-implantitis is similar to that found in patients with advanced periodontal disease1,31. There are studies that show periodontal pathogens colonizing the surface of the implants32,33. Species such as Porphyromonas gingivalis, Prevotella intermedia and Aggregatibacter actinomycetemcomitans collected from advanced periodontal pockets (associated with signs of inflammation) can transfer to peri-implant sites34,35. The presence of particularly virulent biofilm is linked with the appearance of peri-implantitis, depending on the susceptibility of the host and the presence of risk factors (see above). Considering the above, we should ask ourselves if susceptibility to periodontitis increases the susceptibility to peri-implantitis, even in patients who have been treated for periodontitis. Karoussis36 was a pioneer in considering the possibility that people with a history of periodontal disease are more likely to develop peri-implantitis, compared to individuals without a history of periodontal disease.
The analysis of the literature highlights the existence of two categories of studies: a) those researching the success of the therapy in patients with a history of periodontal disease compared to patients who did not suffer from it, and b) those providing a direct comparison between patients with a history of destructive periodontal disease and those with no history of such disease. Several systematic reviews37,38,39,40,41,41,43, as well as a prospective study with an average follow-up period of 10 years, show that people with a history of periodontal disease are more likely to develop peri-implantitis44.
Question No. 5. Which are the therapeutic techniques to treat peri-implantitis and how predictable are they? The therapies proposed to manage peri-implantitis are based on the available evidence from the treatment of periodontal disease. Therefore, the basic steps for resolution are: infection control, surgical and non-surgical therapy, and supportive therapy. To facilitate osseointegration, most implant manufacturers present moderately rough surfaces in the market to increase the contact surface, which would be a disadvantage if the implant is exposed to the oral environment. The roughness of the surface and its chemical composition, as well as the design of threaded implants, have a significant impact on the accumulation of oral biofilm48. The design of the prosthesis can hinder proper mechanical cleaning and treatment of infected implants. Therefore, restoration adjustment is essential for successful patient hygiene49.
Based on the concepts mentioned above, numerous studies suggesting different treatments such as mechanical debridement, use of antiseptics, local and systemic antibiotics as well as surgical access and regenerative procedures have been used, with varying results. The attempts to compare the information available in the literature to draw solid conclusions have failed as there are no data, and because the strict criteria of randomized controlled studies are not met. It is difficult to have randomized controlled trials given the absence of a true control group, the lack of a significant number of patients with similar clinical features, and also for ethical reasons50. A systematic review including nine randomized controlled clinical studies, where researchers tried to identify the most effective treatment, concluded that there was no reliable evidence to suggest which treatment was the best one51. Non-surgical therapy is recommended as the first step. This allows us to evaluate the initial tissue response and the patient's compliance with self-care measures, which will largely determine the success of the therapeutic options proposed. Studies where various forms of non-surgical treatments were compared (mechanical debridement with titanium curettes and polishing with rubber cups vs. mechanical debridement with ultrasonic devices and polishing with rubber cups with antimicrobial administration) were analyzed based on variables such as bleeding on probing, probing depth and biofilm deposits. In the follow-up appointments after one, three and six months, no clinically relevant outcomes were found to support that one treatment is better than the others52. Furthermore, when testing the use of laser devices (Er:YAG)53) and the application of glycine powder at limited pressure54 with mechanical debridement, researchers found no clinically or statistically significant differences. However, non-surgical therapy alone is insufficient in most peri-implantitis cases52. Biofilm and calcified deposits must be removed to allow healing and reduce the risk of disease progression. The surgical protocol that involves surgical access, debridement, decontamination of the surface, irrigation with saline and the use of systemic antibiotics has been evaluated, with positive results over a 12-month period55. On the other hand, studies conducted by Romeo in 2007 mention implantoplasty techniques, i.e. polishing the implant surface with diamond stones, rubbers and silicone materials, repositioning the flap apically56.
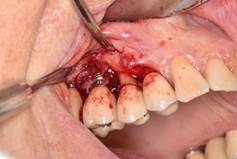
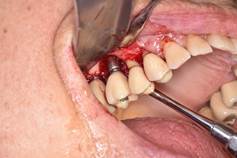
In brief, from the debate held at the peri-implantitis workshop of the First International Congress of Implantology of the School of Dentistry, Universidad de la República, we can conclude that: 1) surgical treatment must always be preceded by non-surgical treatment based on mechanical debridement and the use of antiseptics such as 0.12% chlorhexidine, with a waiting period of three months before starting the surgical treatment (Figs. 8, 9).
Surgical treatment involves: a) opening a mucoperiosteal flap and removing the granulation tissue; b) treating the surface of the implant either with gauze with saline, gauze with 0.2% chlorhexidine, gauze with 3% H2O2, local antibiotics on the implant surface, sodium bicarbonate or glycine spray, Er: YAG laser, mechanical curettage or implantoplasty; c) regenerative or resection techniques; d) systemic antibiotics (amoxicillin 500 mg and metronidazole 500 mg for seven days); e) application of 0.12% chlorhexidine until mechanical hygiene is resumed; f) supportive therapy for three to six months.
The surface treatment techniques above have been analyzed in numerous clinical studies: none has been found to be better than the others. Furthermore, in most cases aesthetic consequences are evident, compromising the success of the prosthetic implant treatment. Although several studies reported short-term results, they also reported no disease resolution, recurrence, and loss of implants despite treatment. Currently there are no certainties regarding the success of medium and long term treatment for peri-implantitis. Further prospective studies that extend over time are necessary to standardize criteria such as definition, disease severity, heterogeneity of design, follow-up period, and inclusion or exclusion criteria.
Regarding maintenance, biofilm must be thoroughly controlled and the patient should be encouraged to do so. In the clinical examination of peri-implant tissues, the professional should probe to control depth, looking for bleeding or suppuration on probing. The prosthesis might need to be removed for this. An occlusal analysis must also be conducted, looking for wear or lack of structure. This must be complemented with antiseptics application57
Referencias
1. Mombelli A, van Oosten MA, Schurch E, Land NP. The microbiota associated with successful or failing osseointegrated titanium implants. Oral Microbiol Immunol. 1987;2:145-51. [ Links ]
2. Albrektsson, T. & Isidor F. Consensus report: implant therapy. Quintessence. 1994;365-369. [ Links ]
3. Zitzmann NU, Berglundh T. Definition and prevalence of peri-implant diseases. J Clin Periodontol. 2008;35:286-91. [ Links ]
4. Berglundh T, Zitzmann NU, M Donati. Are peri-implantitis lesions different from periodontitis lesions? J Clin Periodontol. 2011;38(mar):188 - 202. [ Links ]
5. Froum SJ, Rosen PS. A proposed classification for peri-implantitis. Int J Periodontics Restorative Dent. 2012; 32: 533-40. [ Links ]
6. Luterbacher S, Heitz-Mayfield LJA, Brägger Urs, Lang NP. Diagnostic characteristics of clinical and microbiological tests for monitoring periodontal and peri-implant mucosal tissue conditions during supportive periodontal therapy (SPT). Clin Oral Implant Res. 2000; 11(dec): 521 -529. [ Links ]
7. Hultin M, Gustafsson A, Hallström H, Johansson L-Å, Ekfeldt A, Klinge B. Microbiological findings and host response in patients with peri-implantitis. Clin Oral Implant Res. 2002;13:349-58. [ Links ]
8. Schou S, Holmstrup P, Stoltze K, Hjørting-Hansen E, Kornman K. Ligature-induced marginal inflammation around osseointegrated implants and ankylosed teeth. Clin Oral Implant Res. 1993;4(1):12 - 22. [ Links ]
9. Fransson C, Wennström J, Berglundh T. Clinical characteristics at implants with a history of progressive bone loss. Clin Oral Implants Res. 2008;19:142-7. [ Links ]
10. Etter TH, Håkanson I, Lang NP, Trejo PM, Caffesse RG. Healing after standardized clinical probing of the perlimplant soft tissue seal: a histomorphometric study in dogs. Clin Oral Implants Res. 2002;13:571-80. [ Links ]
11. Lindhe J, Meyle J. Peri-implant diseases: Consensus Report of the Sixth European Workshop on Periodontology. J Clin Periodont 2008: 282-5. [ Links ]
12. Serino G, Turri A, Lang NP. Probing at implants with peri-implantitis and its relation to clinical peri-implant bone loss. Clin Oral Implants Res 2013;24:91-5. [ Links ]
13. Lang NP, Berglundh T. Periimplant diseases: where are we now?--Consensus of the Seventh European Workshop on Periodontology. J Clin Periodontol 2011: 178-81. [ Links ]
14. De Bruyn H, Vandeweghe S, Ruyffelaert C, Cosyn J, Sennerby L. Radiographic evaluation of modern oral implants with emphasis on crestal bone level and relevance to peri-implant health. Periodontol 2000. 2013;62:256-70. [ Links ]
15. Atieh M a, Alsabeeha NHM, Faggion CM, Duncan WJ. The frequency of peri-implant diseases: a systematic review and meta-analysis. J Periodontol 2013;84:1586-98. [ Links ]
16. Berglundh T, Persson L, Klinge B. A systematic review of the incidence of biological and technical complications in implant dentistry reported in prospective longitudinal studies of at least 5 years. J Clin Periodontol. 2002;29 Suppl 3:197-212; discussion 232-233. [ Links ]
17. Heitz-Mayfield LJA. Peri-implant diseases: diagnosis and risk indicators. J Clin Periodontol. 2008;35:292-304. [ Links ]
18. Rocchietta I, Nisand D. A review assessing the quality of reporting of risk factor research in implant dentistry using smoking, diabetes and periodontitis and implant loss as an outcome: critical aspects in design and outcome assessment. J Clin Periodontol 2012;39:114-21. [ Links ]
19. Strietzel FP, Reichart PA, Kale A, Kulkarni M, Wegner B, Küchler I. Smoking interferes with the prognosis of dental implant treatment: a systematic review and meta-analysis. J Clin Periodontol. 2007;34:523-44. [ Links ]
20. Palmer RM, Wilson RF, Hasan AS, Scott DA. Mechanisms of action of environmental factors--tobacco smoking. J Clin Periodontol. 2005;32 Suppl 6:180-95. [ Links ]
21. Ferreira SD, Silva GLM, Cortelli JR, Costa JE, Costa FO. Prevalence and risk variables for peri-implant disease in Brazilian subjects. J Clin Periodontol. 2006;33:929-35. [ Links ]
22. Bornstein MM, Cionca N, Mombelli A. Systemic conditions and treatments as risks for implant therapy. Int J Oral Maxillofac Implants. 2009;24 Suppl:12-27. [ Links ]
23. Academy Report: Peri-Implant Mucositis and Peri-Implantitis: A Current Understanding of Their Diagnoses and Clinical Implications. J Periodontol 2013 Mar 28;84(4):436-43. [ Links ]
24. Fu J-H, Hsu Y-T, Wang H-L. Identifying occlusal overload and how to deal with it to avoid marginal bone loss around implants. Eur J Oral Implantol 2012;5 Suppl:S91-103. [ Links ]
25. Galindo-Moreno P, Fauri M, Avila-Ortiz G, Fernández-Barbero JE, Cabrera-León A, Sánchez-Fernández E. Influence of alcohol and tobacco habits on peri-implant marginal bone loss: a prospective study. Clin Oral Implants Res. 2005;16:579-86. [ Links ]
26. Wilson TG. The positive relationship between excess cement and peri-implant disease: a prospective clinical endoscopic study. J Periodontol. 2009;80:1388-92. [ Links ]
27. Renvert S, Polyzois I, Claffey N. How do implant surface characteristics influence periimplant disease? J Clin Periodont 2011; 38 (suppl 11): 214-22. [ Links ]
28. Gobbato L, Avila-Ortiz G, Sohrabi K, Wang C, Karimbux N. The effect of keratinized mucosa width on peri-implant health: a systematic review. Int J Oral Maxillofac Implant. 2013;28(6):1536-45. [ Links ]
29. Lin G-H, Chan H-L, Wang H-L. The significance of keratinized mucosa on implant health: a systematic review. J Periodontol 2013;84(12):1755-67. [ Links ]
30. Van Steenberghe D, Quirynen M. Reproducibility and detection threshold of peri-implant diagnostics. Adv Dent Res. 1993;7:191-5. [ Links ]
31. Papaioannou W, Quirynen M, Van Steenberghe D. The influence of periodontitis on the subgingival flora around implants in partially edentulous patients. Clin Oral Implants Res. 1996;7:405-9. [ Links ]
32. Leonhardt A, Adolfsson B, Lekholm U, Wikström M, Dahlén G. A longitudinal microbiological study on osseointegrated titanium implants in partially edentulous patients. Clin Oral Implants Res. 1993;4:113-20. [ Links ]
33. Quirynen M, De Soete M, van Steenberghe D. Infectious risks for oral implants: a review of the literature. Clin Oral Implants Res. 2002;13:1-19. [ Links ]
34. Mombelli A, Marxer M, Gaberthüel T, Grunder U, Lang NP. The microbiota of osseointegrated implants in patients with a history of periodontal disease. J Clin Periodontol. 1995;22:124-30. [ Links ]
35. Leonhardt A, Renvert S, Dahlén G. Microbial findings at failing implants. Clin Oral Implants Res. 1999;10:339-45. [ Links ]
36. Karoussis IK, Salvi GE, Heitz-Mayfield LJA, Brägger U, Hämmerle CHF, Lang NP. Long-term implant prognosis in patients with and without a history of chronic periodontitis: a 10-year prospective cohort study of the ITI Dental Implant System. Clin Oral Implants Res. 2003;14:329-39. [ Links ]
37. Van der Weijden GA, van Bemmel KM, Renvert S. Implant therapy in partially edentulous, periodontally compromised patients: a review. J Clin Periodontol. 2005;32:506-11 [ Links ]
38. Schou S, Holmstrup P, Worthington H V, Esposito M. Outcome of implant therapy in patients with previous tooth loss due to periodontitis. Clin Oral Implants Res. 2006;17 Suppl 2:104-23. [ Links ]
39. Karoussis IK, Kotsovilis S, Fourmousis I. A comprehensive and critical review of dental implant prognosis in periodontally compromised partially edentulous patients. Clin Oral Implants Res. 2007;18:669-79. [ Links ]
40. Klokkevold PR, Han TJ. How do smoking, diabetes, and periodontitis affect outcomes of implant treatment? Int J Oral Maxillofac Imp. 2007. p. 173-202. [ Links ]
41. Ong CTT, Ivanovski S, Needleman IG, Retzepi M, Moles DR, Tonetti MS, et al. Systematic review of implant outcomes in treated periodontitis subjects. J Clin Periodontol. 2008;35:438-62. [ Links ]
43. Safii SH, Palmer RM, Wilson RF. Risk of implant failure and marginal bone loss in subjects with a history of periodontitis: A systematic review and meta-analysis. Clin Impl Dent Related Res 2010; 12(3): 165-74. [ Links ]
44. Roccuzzo M, De Angelis N, Bonino L, Aglietta M. Ten-year results of a three-arm prospective cohort study on implants in periodontally compromised patients. Part 1: implant loss and radiographic bone loss. Clin Oral Implants Res. 2010;21:490-6. [ Links ]
45. Tomasi C, Derks J. Clinical research of peri-implant diseases--quality of reporting, case definitions and methods to study incidence, prevalence and risk factors of peri-implant diseases. J Clin Periodontol 2012;39 (Suppl 1):207-23. [ Links ]
46. Mombelli A, Müller N, Cionca N. The epidemiology of peri-implantitis. Clin Oral Implants Res 2012;23 (Suppl 6):67-76. [ Links ]
47. Meijer H, Raghoebar G. Quality of reporting of descriptive studies in implant dentistry. Critical aspects in design, outcome assessment and clinical relevance. J Clin Periodontol 2012; 39(Suppl 12): 108-13. [ Links ]
48. Teughels W, Assche N Van, Sliepen I, Quirynen M. Effect of material characteristics and/or surface topography on biofilm development. Clin Oral Imp Res. 2006; (suppl 6):68-81. [ Links ]
49. Renvert S, Samuelsson E, Lindahl C, Persson GR. Mechanical non-surgical treatment of peri-implantitis: A double-blind randomized longitudinal clinical study. I: Clinical results. J Clin Periodontol. 2009;36:604-9. [ Links ]
50. Heitz-Mayfield LJA, Mombelli A. The Therapy of Peri-implantitis: A Systematic Review. Int J Oral Maxillofac Implants 2014;29 (Suppl):325-45. [ Links ]
51. Esposito M, Grusovin MG, Worthington H V. Treatment of peri-implantitis: what interventions are effective? A Cochrane systematic review. Eur J Oral Implantol 2012;5 (Suppl):S21-41. [ Links ]
52. Renvert S, Roos-Jansåker AM, Claffey N. Non-surgical treatment of peri-implant mucositis and peri-implantitis: A literature review. J Clin Periodontol 2008; 35(Suppl 8): 305-15. [ Links ]
53. Schwarz F, Sculean A, Rothamel D, Schwenzer K, Georg T, Becker J. Clinical evaluation of an Er:YAG laser for nonsurgical treatment of periimplantitis: A pilot study. Clin Oral Implants Res. 2005;16:44-52. [ Links ]
54. Moëne R, Décaillet F, Andersen E, Mombelli A. Subgingival plaque removal using a new air-polishing device. J Periodontol. 2010;81:79-88. [ Links ]
55. Heitz-Mayfield LJA, Salvi GE, Mombelli A, Faddy M, Lang NP. Anti-infective surgical therapy of peri-implantitis. A 12-month prospective clinical study. Clin Oral Implants Res. 2012;23:205-10. [ Links ]
56. Romeo E, Lops D, Chiapasco M, Ghisolfi M, Vogel G. Therapy of peri-implantitis with resective surgery. A 3-year clinical trial on rough screw-shaped oral implants. Part II: Radiographic outcome. Clin Oral Implants Res. 2007;18:179-87. [ Links ]
57. Schwarz F, Sahm N, Iglhaut G, Becker J. Impact of the method of surface debridement and decontamination on the clinical outcome following combined surgical therapy of peri-implantitis: a randomized controlled clinical study. J Clin Periodontol 2011;38:276-84 [ Links ]
Received: March 28, 2017; Accepted: July 11, 2017










 texto em
texto em