Services on Demand
Journal
Article
Related links
Share
Odontoestomatología
Print version ISSN 0797-0374On-line version ISSN 1688-9339
Odontoestomatología vol.17 no.26 Montevideo Nov. 2015
Mandibular myxoma: two clinical cases and a literature review
Kornecki Radzinski, Felipe*
Abstract
The mandibular odontogenic myxoma is a tumor of mesenchymal origin. It represents 0.5 to 17.7 % of odontogenic tumors: the third most frequently occurring type. It is a histologically benign but locally aggressive tumor, with a tendency to recurrence, which determines the treatment followed. In this work, two clinical cases diagnosed with mandibular myxoma are reported: a 33 year-old patient and a 60 year-old patient. Both patients were surgically treated with the segmental mandibulectomy procedure without disarticulation, with safety margins, following Prof. Dr. A Pogrel’s protocol. A mandibular reconstruction appliance was immediately placed, using both an extraoral and an intraoral approach without graft interposition in the first operative time. The tumor characteristics of the two patients with mandibular myxoma and their therapeutic options are discussed.
Keywords: odontogenic myxoma, mandibular reconstruction.
* Dentist. Specialist in Oral and Maxillofacial Surgery and Traumatology. Professor. Department of Oral and Maxillofacial Surgery III. Director of the Postgraduate Degree in Oral and Maxillofacial Surgery and Traumatology. School of Dentistry. Udelar. Certified by the Latin American Board of Oral and Maxillofacial Surgery and Traumatology in 2013.
felipekornecki@gmail.com
Introduction
A myxoma is a benign but locally aggressive tumor, with a tendency to recurrence, which affects the connective tissue. It can be located in soft tissue or bone tissue. Although it is more commonly located in different areas of the head and neck, its most frequent location is in the jaws, where it is known as odontogenic myxoma. It is usually located in the posterior area of the mandible. It most frequently appears in patients in their thirties, and it is less frequent among children and people over 50 (1-7). It is a slow-growing tumor that can grow to a considerable size, and patients often show no symptoms. Some authors describe the presence of paresthesia and pain.
As it grows in size, it expands the cortical bone and can even cause tooth displacement, hence modifying occlusion (8). It originates from embryonic mesenchymal tissue of the tooth germ, papillae, follicle and periodontal ligament. It is the third most frequently occurring type after the odontoma and the amelobastoma. Anatomic pathology of this neoplasm shows fusiform cells in a myxoid stroma. As it has no capsule, this tumor may penetrate the trabecular areas, destroying the cortical bone and perforating and invading adjacent soft tissues. This capacity to invade the bone is connected to a high risk of recurrence. Most authors agree that a myxoma should be treated through radical surgical resection with radiological safety margins of at least 0.5 to 1 cm.
Clinical case 1
A 33-year-old patient with no relevant personal history that sets an appointment with his dentist in 2006 on account of a swelling in the right lower area of his jaw.
The professional makes the clinical and radiological diagnosis of the third retained lower molar (4.8) with an associated radiolucent lesion (Fig.1).
The dentist prescribes the extraction of tooth 4.8 but the patient does not comply with the professional's instructions.

Fig.1. Radiolucent lesion associated with molar 4.8 (2006)
In 2007, one year after the first appointment, the patient sees his dentist again. The professional orders a new panoramic radiograph, and when comparing both OPTs, he notices an increase in the size of the distal radiolucent area in the third lower molar. At the time, the radiolucent area spread from the distal area of the third lower molar to the base of the mandibular condyle and of the coronoid apophysis (Fig. 2).
The patient is referred to the Oral and Maxillofacial Surgeon and a biopsy is ordered.

Fig. 2 Radiolucent area associated to molar 4.8 (2007)
In 2007, the biopsy is done and the anatomic pathology report confirms the diagnosis: mandibular odontogenic myxoma. A treatment plan is devised, routine preoperative tests and a CT are ordered (Fig. 3).

Fig. 3 CT, frontal and horizontal sections
Treatment. First surgery.
The internal surgical field is prepared under general anesthesia and nasotracheal intubation, and pharyngeal packing is placed. Intermaxillary fixation is placed using 0.5 mm intermaxillary fixation screws and wires. The area is infiltrated with 2% mepivacaine. Surgery begins intraorally, with a vestibular and lingual incision that includes teeth 4.6 and 4.7. The incision goes up along the anterior edge of the ramus, considering the planned margins. Subperiostal degloving is performed at tooth 4.4, and a 1 cm margin above the tumor is sought in the ramus. Osteotomy begins through this intraoral access with a number 8 bur. Physiological saline solution is applied on the proximal and distal ends of the lesion.
The procedure continues extraorally in the area below the mandibular angle. The mandible is accessed through preseptal dissection, ligating the facial bundle, artery and vein, respecting and preserving the mandibular nerve, ramus of the facial nerve.
Before completing the osteotomy, 2.3 parallel reconstruction plates are placed (Fig. 4). Their position is set using the screws once the mandibular resection is complete.

Fig. 4 parallel plates 2.3 in position
The plates are then removed and the osteotomy is completed. The mandibular tumor is resected without disarticulation. Reconstruction is performed with two 2.3 parallel plates.
Double layer suturing is used to close (Fig. 5: A, B and C).

Fig. 5 A- Intermaxillary fixation
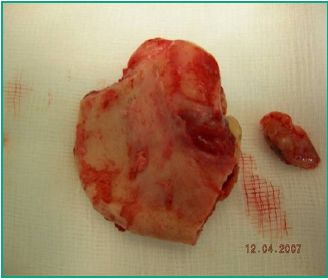
Fig. 5. B- Surgical specimen

Fig. 5. C- Reconstruction plates in position
The patient's evolution is monitored at the Clinic of Oral and Maxillofacial Surgery and Traumatology at Servicio Médico Integral (SMI). He evolves favorably.
Clinical monitoring, Fig 6. A, B, C and D.

Fig. 6. A. Occlusion with mouth closed

Fig. 6. B. Evaluation of cervical approach

Fig. 6. C and D. Evaluation of occlusion and facial midline
Fig. 7 A and B show postoperative control of the first surgery by OPT and CT scan.

Fig. 7. A. Control panoramic radiograph

Fig. 7. B. Control CT scan
The results of the histopathological study of the surgical specimen are received, confirming the diagnosis of mandibular myxoma (Fig. 8).

Fig. 8. Histopathological report
Second surgery. A 3D CT scan with reconstruction on the three planes is ordered (Fig. 9 A and B).

Fig. 9. A

Fig. 9. B
Given the mesio-distal extension of the lesion, an iliac crest graft is harvested as the defect is larger than 7 cm. The graft is designed and the traumatologist approaches the patient in the ventral decubitus position, harvests posterior iliac crest graft, begins hemostasis in the area, closes and places suction drain (Fig. 10: A, B, C and D).

Fig. 10 A. Posterior iliac crest graft harvest

Fig. 10. B. Particulate graft harvest

Fig. 10. C. Graft carving

Fig. 10. D. Suture of posterior approach and placement of drain
The patient is placed in dorsal decubitus position. The area operated on is approached again from the neck. The graft is shaped with a bur under physiological saline solution, it is adapted and fixed to the mandibular reconstruction plate of the first surgery (Fig.11).

Fig. 11 Graft in place
Clinical examination and imaging tests show the patient evolves favorably (Fig. 12: A and B).

Fig. 12 A. Evaluation of occlusion

Fig 12 B. Evaluation of cervical approach
Clinical case 2.
A 60-year-old male, married with children, from the Dept. of Artigas, Uruguay. He has a history of high blood pressure and is a smoker. He has had a swelling in the posterior left mandible area for several years. He was diagnosed with mandibular myxoma via a biopsy. Patient provides imaging tests (OPT scan) ordered by treating dentist (Fig. 13).

Fig. 13. Preoperative OPT
A CAT scan is ordered with sections in all three planes to complement the tests necessary for the surgical treatment plan (Fig. 14 and 15).

Fig. 14. CAT scan (frontal sections)

Fig. 15. CAT scan (sagittal section)
Preoperative tests are ordered, and patient is referred to an internist and an anesthesiologist. A surgical procedure under general anesthesia and nasotracheal intubation is scheduled.
Treatment. First surgery.
The following is the surgical plan followed according to the lesion extension determined clinically and through imaging tests:
- Intraoral approach of the front edge of the ascending ramus of the mandible.
- Subperiosteal dissection of inner surface of ramus.
- Lower alveolar bundle is clamped and cut.
- Avulsion of tooth 33 for safety margin. The ramus direction is followed upwards up to 1 cm above the lesion.
- Cervical approach. Dissection by layers until the mandibular plane is reached.
- Facial bundle is ligated and the mandibular nerve is preserved.
- The position of the mandibular reconstruction plate is adapted and delimited.
- Segmental mandibulectomy from alveolus of tooth 33, from the center to the base of the coronoid apophysis and the mandibular condyle using an oscillating saw (Fig. 16).
- Final shaping and fixation of reconstruction plate using cortico-cortical screws (Fig. 16 A and B).
- Ligation of inferior dental bundle and removal of hemostat.
- Intra and extraoral double layer suturing.

Fig. 16. Surgical specimen

Fig. 16 A and B. Cervical approach and placement of 2.4 reconstruction plate.
Evolution is monitored via CT before second surgery (Fig.17). The frontal view shows the misalignment of the first reconstruction plate (Fig. 18).

Fig. 17. Control CAT scan

Fig. 18. Frontal CAT scan
Second surgery
The traumatologist harvests an anterior iliac crest graft while the patient is under general anesthesia and nasotracheal intubation. The same cervical approach is taken along the anterior scar line (Fig. 19). Dissection by layers until the reconstruction plate from the first surgery is reached. While the patient is in occlusion, the initial plate is removed using intermaxillary fixation screws and wires, and a new 2.4 plate is adapted. Work is done on the graft harvested with burs under physiological saline solution irrigation. The surgical field is adapted and fixed to the mesial and distal ends of the resection using 2.0 low profile plates. A 2.4 plate is placed over the graft (Fig 20). The wound is cleansed and closed by layers using Vicryl 3.0 and RV needle (Fig. 21). An X-ray is taken (Fig. 22 and 23).

Fig.19. Cervical scar from first surgery

Fig. 20. Removal of initial plate. Graft adaptation and fixation of ends to resection with mesial and distal 2.0 plates.

Fig. 21. Superficial closure of layers.

Fig. 22. Postoperative X-ray evaluation (frontal)

Fig. 23. Postoperative X-ray evaluation (lateral)
Discussion
An odontogenic myxoma is a benign but aggressive tumor, with a tendency to recurrence and infiltration after inadequate surgical resection. As it has no capsule, the tumor may penetrate bone trabecular areas, which makes it mandatory to control safety margins. Mixomas are treated surgically, following the different protocols in the literature. Ideal treatment uses a microvascular graft.
Advantages of microsurgical reconstruction
- Primary reconstruction with a high success rate
- Fewer complications
- Design flexibility
- Increase of tumor resectability rates
- Minimal aesthetic impact and functionality at donor site
Disadvantages of microsurgical reconstruction
- It requires a specially trained team
- It is necessary to find a recipient artery and a recipient vein
- Costly procedure
- Length of surgery
Microsurgery indications
- Soft tissue reconstruction
- Complex craniofacial resections
- Mandible and maxillary reconstruction
- Some cases of facial paralysis
- Hemifacial microsomia
- Full nose reconstruction
Iliac crest and fibula grafts are the most frequently used bone grafts. When microvascularized iliac crest is used, dental implants can be placed in the same procedure (2, 3), as the iliac crest provides the necessary width and height for immediate reconstruction. If a fibula graft is used, it can be used as it is, taken from the donor site (simple); however, there are two issues that do not make immediate reconstruction possible (2, 3): it does not provide the necessary height for immediate reconstruction using osseointegration implants (9), and the use of fixation means when shaping the mandibular arch with the fibula makes subsequent implant placement difficult (10). We can use the double-barrel technique for the fibula graft, which can endanger its vascularization, or we can perform osteogenic distraction after its local adaptation (1, 6).
Prof. Dr. A Pogrel’s protocol (11) was used to treat the patients focus of this study. It involves the segmental resection of the area which is pathologically compromised, and immediate reconstruction with a reconstruction plate through an intra and extraoral approach.
Prof. Dr. A. Pogret's protocol
1. Autogenous graft secondary to the first surgery in 6 to 8 weeks through an extraoral approach
2. Removal of reconstruction plate 4 to 6 months after placing the autogenous graft
3. Skin graft and vestibuloplasty 6 to 8 weeks after removing the plate
4. Osseointegration implants 8 to 12 weeks after vestibuloplasty
5. Second stage of implants 4 to 6 months after placing the first implants
6. Prosthetic reconstruction
Conclusions
This work describes the treatment of two male patients, aged 33 and 60 respectively, who had an odontogenic myxoma located in the posterior area of the mandible. Both patients underwent mandibulectomy without disarticulation, a radical surgical procedure. Reconstruction was done using 2.3 mm and 2.4 mm titanium mandibular reconstruction plates. In the second surgery, six months after the initial procedure, a posterior iliac crest graft was placed on the first patient and an anterior iliac crest graft on the second patient. Both patients have evolved well as shown in the clinical and imaging evaluations. The first patient can already begin the rehabilitation process but has not done so yet. The second patient awaits the evolution of the graft.
References
1.Navarro Cuéllar C, Cebrian Carretero JL, Garcia Rozado González A. Reconstrucción Mandibular. In: Sociedad Española de Cirugía Oral y Maxilofacial. Protocolos Clínicos de la SECOM. 2014. Chapter 45. Available from:
www.secom.org/web/wp-content/uploads/2014/01/cap45.pdf
2.Castro AL de, Kanno CM, Callestini R, Goncalves L, Sicchieri LG, Munhoz FC. Mixoma Odontogénico em Mandíbula. Rev Odontol Araçatuba 2003; 24(2): 23-27.
3.Zhi-Min Liu, Di Wu, Xue-Kui Liu, Wei-Wei Liu, Hao Li, Quan Li, Xin-Rui Zhang, Zong-Yuan Zeng. Application of anteromedial thigh flap for the reconstruction of oral and maxillofacial defects. J Oral Maxillofac Surg 71: 964, 2013:
4.Zhao L, Shang H, Chen X, Liu Y. Biomechanical analysis of a curvilinear distractor device for correcting mandibular symphyseal defects. J Oral Maxillofac Surg 2014; 72(6):1158-67.
5.Kenke E, Agaimy A, Von Wilmowsky C, Eitner S. Mandibular reconstruction using intraoral microvascular anastomosis following removal of an ameloblastoma.. J Oral Maxillofac Surg 2013; 71(11): 1983-1992
6.Kansy K, Juergens P, Krol Z, Paulussen M, Baumhoer D, Bruder E, Schneider J, Zeilhofer HF, Schwenzer-Zimmerer K. Odontogenic myxoma: diagnostic and therapeutic challenges in paediatric and adult patients-a case series and review of the literature. J Craniomaxillofac Surg 2012; 40(3):271-276
7.Abdennour S, Benhalima H. Les tumeurs odontogènes bénignes: analyse épidémiologique de 97 cas dans la population algérienne. Rev Stomatol Chir Maxillo-Fac Chir Orale. 2013; 114(2): 67-71
8.Parr J, Adams BM, Wagels M. Flow-through flap for salvage of fibula osseocutaneous vascular variations: A surgical approach and proposed modification of its classification. J Oral Maxillofac Surg 2014; 72: 1197-1202
9.Hupp J. Ellis III E, Tucker M. Cirugía Oral y Maxilofacial Contemporanea. 6th edition. Barcelona: Elsevier-Mosby, 2014.
10.Navarro Cuéllar C, Ochandiano Caicoya S, Riba García F, López de Atalaya FJ, Acero Sanz J, Cuesta Gil M, Navarro Vila C. Rehabilitación implanto soportada en el colgajo libre de peroné. Rev. Esp. Cir. Oral Maxilofac 2006; 28(5): 263-275
11.Pogrel MA, Podles S, Anthony JP, Alexander J. A comparison of vascularized and nonvascularized bone grafts for reconstruction of mandibular continuity defects. J Oral Maxillofac Surg 1997; 55(11): 1200-11.
.
Kornecki Radzinski, Felipe*
Abstract
The mandibular odontogenic myxoma is a tumor of mesenchymal origin. It represents 0.5 to 17.7 % of odontogenic tumors: the third most frequently occurring type. It is a histologically benign but locally aggressive tumor, with a tendency to recurrence, which determines the treatment followed. In this work, two clinical cases diagnosed with mandibular myxoma are reported: a 33 year-old patient and a 60 year-old patient. Both patients were surgically treated with the segmental mandibulectomy procedure without disarticulation, with safety margins, following Prof. Dr. A Pogrel’s protocol. A mandibular reconstruction appliance was immediately placed, using both an extraoral and an intraoral approach without graft interposition in the first operative time. The tumor characteristics of the two patients with mandibular myxoma and their therapeutic options are discussed.
Keywords: odontogenic myxoma, mandibular reconstruction.
* Dentist. Specialist in Oral and Maxillofacial Surgery and Traumatology. Professor. Department of Oral and Maxillofacial Surgery III. Director of the Postgraduate Degree in Oral and Maxillofacial Surgery and Traumatology. School of Dentistry. Udelar. Certified by the Latin American Board of Oral and Maxillofacial Surgery and Traumatology in 2013.
felipekornecki@gmail.com
Introduction
A myxoma is a benign but locally aggressive tumor, with a tendency to recurrence, which affects the connective tissue. It can be located in soft tissue or bone tissue. Although it is more commonly located in different areas of the head and neck, its most frequent location is in the jaws, where it is known as odontogenic myxoma. It is usually located in the posterior area of the mandible. It most frequently appears in patients in their thirties, and it is less frequent among children and people over 50 (1-7). It is a slow-growing tumor that can grow to a considerable size, and patients often show no symptoms. Some authors describe the presence of paresthesia and pain.
As it grows in size, it expands the cortical bone and can even cause tooth displacement, hence modifying occlusion (8). It originates from embryonic mesenchymal tissue of the tooth germ, papillae, follicle and periodontal ligament. It is the third most frequently occurring type after the odontoma and the amelobastoma. Anatomic pathology of this neoplasm shows fusiform cells in a myxoid stroma. As it has no capsule, this tumor may penetrate the trabecular areas, destroying the cortical bone and perforating and invading adjacent soft tissues. This capacity to invade the bone is connected to a high risk of recurrence. Most authors agree that a myxoma should be treated through radical surgical resection with radiological safety margins of at least 0.5 to 1 cm.
Clinical case 1
A 33-year-old patient with no relevant personal history that sets an appointment with his dentist in 2006 on account of a swelling in the right lower area of his jaw.
The professional makes the clinical and radiological diagnosis of the third retained lower molar (4.8) with an associated radiolucent lesion (Fig.1).
The dentist prescribes the extraction of tooth 4.8 but the patient does not comply with the professional's instructions.

Fig.1. Radiolucent lesion associated with molar 4.8 (2006)
In 2007, one year after the first appointment, the patient sees his dentist again. The professional orders a new panoramic radiograph, and when comparing both OPTs, he notices an increase in the size of the distal radiolucent area in the third lower molar. At the time, the radiolucent area spread from the distal area of the third lower molar to the base of the mandibular condyle and of the coronoid apophysis (Fig. 2).
The patient is referred to the Oral and Maxillofacial Surgeon and a biopsy is ordered.

Fig. 2 Radiolucent area associated to molar 4.8 (2007)
In 2007, the biopsy is done and the anatomic pathology report confirms the diagnosis: mandibular odontogenic myxoma. A treatment plan is devised, routine preoperative tests and a CT are ordered (Fig. 3).

Fig. 3 CT, frontal and horizontal sections
Treatment. First surgery.
The internal surgical field is prepared under general anesthesia and nasotracheal intubation, and pharyngeal packing is placed. Intermaxillary fixation is placed using 0.5 mm intermaxillary fixation screws and wires. The area is infiltrated with 2% mepivacaine. Surgery begins intraorally, with a vestibular and lingual incision that includes teeth 4.6 and 4.7. The incision goes up along the anterior edge of the ramus, considering the planned margins. Subperiostal degloving is performed at tooth 4.4, and a 1 cm margin above the tumor is sought in the ramus. Osteotomy begins through this intraoral access with a number 8 bur. Physiological saline solution is applied on the proximal and distal ends of the lesion.
The procedure continues extraorally in the area below the mandibular angle. The mandible is accessed through preseptal dissection, ligating the facial bundle, artery and vein, respecting and preserving the mandibular nerve, ramus of the facial nerve.
Before completing the osteotomy, 2.3 parallel reconstruction plates are placed (Fig. 4). Their position is set using the screws once the mandibular resection is complete.

Fig. 4 parallel plates 2.3 in position
The plates are then removed and the osteotomy is completed. The mandibular tumor is resected without disarticulation. Reconstruction is performed with two 2.3 parallel plates.
Double layer suturing is used to close (Fig. 5: A, B and C).

Fig. 5 A- Intermaxillary fixation

Fig. 5. B- Surgical specimen

Fig. 5. C- Reconstruction plates in position
The patient's evolution is monitored at the Clinic of Oral and Maxillofacial Surgery and Traumatology at Servicio Médico Integral (SMI). He evolves favorably.
Clinical monitoring, Fig 6. A, B, C and D.

Fig. 6. A. Occlusion with mouth closed

Fig. 6. B. Evaluation of cervical approach

Fig. 6. C and D. Evaluation of occlusion and facial midline
Fig. 7 A and B show postoperative control of the first surgery by OPT and CT scan.

Fig. 7. A. Control panoramic radiograph

Fig. 7. B. Control CT scan
The results of the histopathological study of the surgical specimen are received, confirming the diagnosis of mandibular myxoma (Fig. 8).

Fig. 8. Histopathological report
Second surgery. A 3D CT scan with reconstruction on the three planes is ordered (Fig. 9 A and B).

Fig. 9. A

Fig. 9. B
Given the mesio-distal extension of the lesion, an iliac crest graft is harvested as the defect is larger than 7 cm. The graft is designed and the traumatologist approaches the patient in the ventral decubitus position, harvests posterior iliac crest graft, begins hemostasis in the area, closes and places suction drain (Fig. 10: A, B, C and D).

Fig. 10 A. Posterior iliac crest graft harvest

Fig. 10. B. Particulate graft harvest

Fig. 10. C. Graft carving

Fig. 10. D. Suture of posterior approach and placement of drain
The patient is placed in dorsal decubitus position. The area operated on is approached again from the neck. The graft is shaped with a bur under physiological saline solution, it is adapted and fixed to the mandibular reconstruction plate of the first surgery (Fig.11).

Fig. 11 Graft in place
Clinical examination and imaging tests show the patient evolves favorably (Fig. 12: A and B).

Fig. 12 A. Evaluation of occlusion

Fig 12 B. Evaluation of cervical approach
Clinical case 2.
A 60-year-old male, married with children, from the Dept. of Artigas, Uruguay. He has a history of high blood pressure and is a smoker. He has had a swelling in the posterior left mandible area for several years. He was diagnosed with mandibular myxoma via a biopsy. Patient provides imaging tests (OPT scan) ordered by treating dentist (Fig. 13).

Fig. 13. Preoperative OPT
A CAT scan is ordered with sections in all three planes to complement the tests necessary for the surgical treatment plan (Fig. 14 and 15).

Fig. 14. CAT scan (frontal sections)

Fig. 15. CAT scan (sagittal section)
Preoperative tests are ordered, and patient is referred to an internist and an anesthesiologist. A surgical procedure under general anesthesia and nasotracheal intubation is scheduled.
Treatment. First surgery.
The following is the surgical plan followed according to the lesion extension determined clinically and through imaging tests:
- Intraoral approach of the front edge of the ascending ramus of the mandible.
- Subperiosteal dissection of inner surface of ramus.
- Lower alveolar bundle is clamped and cut.
- Avulsion of tooth 33 for safety margin. The ramus direction is followed upwards up to 1 cm above the lesion.
- Cervical approach. Dissection by layers until the mandibular plane is reached.
- Facial bundle is ligated and the mandibular nerve is preserved.
- The position of the mandibular reconstruction plate is adapted and delimited.
- Segmental mandibulectomy from alveolus of tooth 33, from the center to the base of the coronoid apophysis and the mandibular condyle using an oscillating saw (Fig. 16).
- Final shaping and fixation of reconstruction plate using cortico-cortical screws (Fig. 16 A and B).
- Ligation of inferior dental bundle and removal of hemostat.
- Intra and extraoral double layer suturing.

Fig. 16. Surgical specimen

Fig. 16 A and B. Cervical approach and placement of 2.4 reconstruction plate.
Evolution is monitored via CT before second surgery (Fig.17). The frontal view shows the misalignment of the first reconstruction plate (Fig. 18).

Fig. 17. Control CAT scan

Fig. 18. Frontal CAT scan
Second surgery
The traumatologist harvests an anterior iliac crest graft while the patient is under general anesthesia and nasotracheal intubation. The same cervical approach is taken along the anterior scar line (Fig. 19). Dissection by layers until the reconstruction plate from the first surgery is reached. While the patient is in occlusion, the initial plate is removed using intermaxillary fixation screws and wires, and a new 2.4 plate is adapted. Work is done on the graft harvested with burs under physiological saline solution irrigation. The surgical field is adapted and fixed to the mesial and distal ends of the resection using 2.0 low profile plates. A 2.4 plate is placed over the graft (Fig 20). The wound is cleansed and closed by layers using Vicryl 3.0 and RV needle (Fig. 21). An X-ray is taken (Fig. 22 and 23).

Fig.19. Cervical scar from first surgery

Fig. 20. Removal of initial plate. Graft adaptation and fixation of ends to resection with mesial and distal 2.0 plates.

Fig. 21. Superficial closure of layers.

Fig. 22. Postoperative X-ray evaluation (frontal)

Fig. 23. Postoperative X-ray evaluation (lateral)
Discussion
An odontogenic myxoma is a benign but aggressive tumor, with a tendency to recurrence and infiltration after inadequate surgical resection. As it has no capsule, the tumor may penetrate bone trabecular areas, which makes it mandatory to control safety margins. Mixomas are treated surgically, following the different protocols in the literature. Ideal treatment uses a microvascular graft.
Advantages of microsurgical reconstruction
- Primary reconstruction with a high success rate
- Fewer complications
- Design flexibility
- Increase of tumor resectability rates
- Minimal aesthetic impact and functionality at donor site
Disadvantages of microsurgical reconstruction
- It requires a specially trained team
- It is necessary to find a recipient artery and a recipient vein
- Costly procedure
- Length of surgery
Microsurgery indications
- Soft tissue reconstruction
- Complex craniofacial resections
- Mandible and maxillary reconstruction
- Some cases of facial paralysis
- Hemifacial microsomia
- Full nose reconstruction
Iliac crest and fibula grafts are the most frequently used bone grafts. When microvascularized iliac crest is used, dental implants can be placed in the same procedure (2, 3), as the iliac crest provides the necessary width and height for immediate reconstruction. If a fibula graft is used, it can be used as it is, taken from the donor site (simple); however, there are two issues that do not make immediate reconstruction possible (2, 3): it does not provide the necessary height for immediate reconstruction using osseointegration implants (9), and the use of fixation means when shaping the mandibular arch with the fibula makes subsequent implant placement difficult (10). We can use the double-barrel technique for the fibula graft, which can endanger its vascularization, or we can perform osteogenic distraction after its local adaptation (1, 6).
Prof. Dr. A Pogrel’s protocol (11) was used to treat the patients focus of this study. It involves the segmental resection of the area which is pathologically compromised, and immediate reconstruction with a reconstruction plate through an intra and extraoral approach.
Prof. Dr. A. Pogret's protocol
1. Autogenous graft secondary to the first surgery in 6 to 8 weeks through an extraoral approach
2. Removal of reconstruction plate 4 to 6 months after placing the autogenous graft
3. Skin graft and vestibuloplasty 6 to 8 weeks after removing the plate
4. Osseointegration implants 8 to 12 weeks after vestibuloplasty
5. Second stage of implants 4 to 6 months after placing the first implants
6. Prosthetic reconstruction
Conclusions
This work describes the treatment of two male patients, aged 33 and 60 respectively, who had an odontogenic myxoma located in the posterior area of the mandible. Both patients underwent mandibulectomy without disarticulation, a radical surgical procedure. Reconstruction was done using 2.3 mm and 2.4 mm titanium mandibular reconstruction plates. In the second surgery, six months after the initial procedure, a posterior iliac crest graft was placed on the first patient and an anterior iliac crest graft on the second patient. Both patients have evolved well as shown in the clinical and imaging evaluations. The first patient can already begin the rehabilitation process but has not done so yet. The second patient awaits the evolution of the graft.
References
1.Navarro Cuéllar C, Cebrian Carretero JL, Garcia Rozado González A. Reconstrucción Mandibular. In: Sociedad Española de Cirugía Oral y Maxilofacial. Protocolos Clínicos de la SECOM. 2014. Chapter 45. Available from:
www.secom.org/web/wp-content/uploads/2014/01/cap45.pdf
2.Castro AL de, Kanno CM, Callestini R, Goncalves L, Sicchieri LG, Munhoz FC. Mixoma Odontogénico em Mandíbula. Rev Odontol Araçatuba 2003; 24(2): 23-27.
3.Zhi-Min Liu, Di Wu, Xue-Kui Liu, Wei-Wei Liu, Hao Li, Quan Li, Xin-Rui Zhang, Zong-Yuan Zeng. Application of anteromedial thigh flap for the reconstruction of oral and maxillofacial defects. J Oral Maxillofac Surg 71: 964, 2013:
4.Zhao L, Shang H, Chen X, Liu Y. Biomechanical analysis of a curvilinear distractor device for correcting mandibular symphyseal defects. J Oral Maxillofac Surg 2014; 72(6):1158-67.
5.Kenke E, Agaimy A, Von Wilmowsky C, Eitner S. Mandibular reconstruction using intraoral microvascular anastomosis following removal of an ameloblastoma.. J Oral Maxillofac Surg 2013; 71(11): 1983-1992
6.Kansy K, Juergens P, Krol Z, Paulussen M, Baumhoer D, Bruder E, Schneider J, Zeilhofer HF, Schwenzer-Zimmerer K. Odontogenic myxoma: diagnostic and therapeutic challenges in paediatric and adult patients-a case series and review of the literature. J Craniomaxillofac Surg 2012; 40(3):271-276
7.Abdennour S, Benhalima H. Les tumeurs odontogènes bénignes: analyse épidémiologique de 97 cas dans la population algérienne. Rev Stomatol Chir Maxillo-Fac Chir Orale. 2013; 114(2): 67-71
8.Parr J, Adams BM, Wagels M. Flow-through flap for salvage of fibula osseocutaneous vascular variations: A surgical approach and proposed modification of its classification. J Oral Maxillofac Surg 2014; 72: 1197-1202
9.Hupp J. Ellis III E, Tucker M. Cirugía Oral y Maxilofacial Contemporanea. 6th edition. Barcelona: Elsevier-Mosby, 2014.
10.Navarro Cuéllar C, Ochandiano Caicoya S, Riba García F, López de Atalaya FJ, Acero Sanz J, Cuesta Gil M, Navarro Vila C. Rehabilitación implanto soportada en el colgajo libre de peroné. Rev. Esp. Cir. Oral Maxilofac 2006; 28(5): 263-275
11.Pogrel MA, Podles S, Anthony JP, Alexander J. A comparison of vascularized and nonvascularized bone grafts for reconstruction of mandibular continuity defects. J Oral Maxillofac Surg 1997; 55(11): 1200-11.
.
2.Castro AL de, Kanno CM, Callestini R, Goncalves L, Sicchieri LG, Munhoz FC. Mixoma Odontogénico em Mandíbula. Rev Odontol Aracatuba 2003; 24(2): 23-27.
3.Zhi-Min Liu, Di Wu, Xue-Kui Liu, Wei-Wei Liu, Hao Li, Quan Li, Xin-Rui Zhang, Zong-Yuan Zeng. Application of anteromedial thigh flap for the reconstruction of oral and maxillofacial defects. J Oral Maxillofac Surg 71: 964, 2013.
4.Zhao L, Shang H, Chen X, Liu Y. Biomechanical analysis of a curvilinear distractor device for correcting mandibular symphyseal defects. J Oral Maxillofac Surg 2014; 72(6):1158-67.
5.Kenke E, Agaimy A, Von Wilmowsky C, Eitner S. Mandibular reconstruction using intraoral microvascular anastomosis following removal of an ameloblastoma.. J Oral Maxillofac Surg 2013; 71(11): 1983-1992
6.Kansy K, Juergens P, Krol Z, Paulussen M, Baumhoer D, Bruder E, Schneider J, Zeilhofer HF, Schwenzer-Zimmerer K. Odontogenic myxoma: diagnostic and therapeutic challenges in paediatric and adult patients-a case series and review of the literature. J Craniomaxillofac Surg 2012; 40(3):271-276
7.Abdennour S, Benhalima H. Les tumeurs odontogènes bénignes: analyse épidémiologique de 97 cas dans la population algérienne. Rev Stomatol Chir Maxillo-Fac Chir Orale. 2013; 114(2): 67-71
8.Parr J, Adams BM, Wagels M. Flow-through flap for salvage of fibula osseocutaneous vascular variations: A surgical approach and proposed modification of its classification. J Oral Maxillofac Surg 2014; 72: 1197-1202
9.Hupp J. Ellis III E, Tucker M. Cirugía Oral y Maxilofacial Contemporanea. 6ed. Barcelona: Elsevier-Mosby, 2014.
10.Navarro Cuéllar C, Ochandiano Caicoya S, Riba García F, López de Atalaya FJ, Acero Sanz J, Cuesta Gil M, Navarro Vila C. Rehabilitación implanto soportada en el colgajo libre de peroné. Rev. Esp. Cir. Oral Maxilofac 2006; 28(5): 263-275
11.Pogrel MA, Podles S, Anthony JP, Alexander J. A comparison of vascularized and nonvascularized bone grafts for reconstruction of mandibular continuity defects. J Oral Maxillofac Surg 1997; 55(11): 1200-11.











 text in
text in 


