Services on Demand
Journal
Article
Related links
Share
Odontoestomatología
Print version ISSN 0797-0374On-line version ISSN 1688-9339
Odontoestomatología vol.17 no.25 Montevideo May 2015
Immunohistochemical analysis of CK14 and CK19 in tooth germ and ameloblastoma
Apellaniz, Delmira*, Nieves, Sabrina*, Tapia, Gabriel**, Maglia, Alvaro***, Mosqueda-Taylor, Adalberto****, Bologna-Molina, Ronell*****
* Molecular Pathology Trainee, School of Dentistry, Universidad de la República. Uruguay.
**
Associate Professor, Grade 3, Histology Department, School of Dentistry, Universidad de la República. Uruguay
*** Professor, Grade 5, Histology Department, School of Dentistry, Universidad de la República. Uruguay
**** Specialist in Pathology and Oral Medicine, Master’s Degree in Oral Pathology, Full-time Research Professor, Health Care Department, Universidad Autónoma Metropolitana, Mexico
***** Specialist in Pathology and Oral Medicine, Doctor in Biological Sciences (Molecular Pathology), Professor, Grade 5, Molecular Pathology, School of Dentistry, Universidad de la República. Uruguay. ronllbologna@hotmail.com
Abstract
Introduction. Odontogenesis is the process by which teeth form, and where different molecules are expressed, among them some cytokeratins (CK) like CK14 and CK19. Remnants of odontogenic epithelium may persist once the development process is complete, which has been suggested to be involved in the development of ameloblastoma, one of the most common benign odontogenic tumors. It has been suggested that CK14 and CK19 are useful markers of ameloblast differentiation and that they could have implications for tumor behavior. The aim of this study was to describe the patterns of immunohistochemical expression of these cytokeratins in tooth germs and ameloblastomas.
Materials and methods. We worked with 6 solid multicystic ameloblastomas and 5 tooth germs. The immunohistochemistry technique was used to visualize CK14 and CK19.
Results. We detected CK14 and CK19 immunoexpression in the epithelium and no expression in the ectomesenchyme in both tooth germs and ameloblastomas. It was concluded that CK19 can be considered an efficient marker of ameloblast differentiation, whereas CK14 is gradually replaced by CK19 in the inner epithelium of the enamel organ, showing strong immunoexpression in secretory ameloblasts.
Keywords: odontogenesis, tooth germ, ameloblastoma, cytokeratins 14 and 19.
Received on: 08.05.14 - Accepted on: 02.02.15
Introduction
Odontogenesis is a biological process by which stomodeal ectodermal cells interact with underlying ectomesenchymal cells to form teeth. Tooth development includes growth, migration and cell differentiation, as well as a series of events that result from a complex and intricate cascade of genes that are expressed in a specific place and sequence (1).
Several molecules are expressed both in the ectomesenchyme and in the ectoderm, and they have different roles (2). Good examples of this are the cytokeratins expressed by epithelial cells and which are responsible for diverse biological mechanisms where epithelia participate (3-6). Once odontogenesis is complete, there are areas in maxilla and mandible where we can find remnants of odontogenic epithelium (7). It has been suggested that these remnants can give rise to various lesions such as cysts and odontogenic tumors. The most frequent odontogenic tumor is the ameloblastoma, a locally aggressive benign neoplasm which has a wide spectrum of histological patterns that resemble the early stages of odontogenesis (8).
The aim of this study was to describe the patterns of immunohistochemical expression of two cytokeratins, CK14 and CK19, in tooth germs and ameloblastomas. As the expression of these two cytokeratins has been shown in odontogenesis and carcinogenesis, and its usefulness as a prognosis tool in various malignant neoplasms has been proven, we believe that it is important to try to understand the role of these intermediate filaments in embryonic and tumor progression processes (9). This is why we decided to study these two cytokeratins in normal embryonic processes (odontogenesis) and in pathological neoplastic processes (ameloblastoma).
Materials and methods
For the immunohistochemical study we worked with 6 solid multicystic ameloblastomas from the Oral Pathology Lab of Universidad Autónoma Metropolitana Xochimilco (Mexico) and 5 tooth germs from the Histology Department of the School of Dentistry, Universidad de la República (Uruguay). Both tumors and tooth germs were stored and placed in paraffin blocks. A descriptive-observational study was conducted.
Immunohistochemistry technique
Sections measuring 2-μm were cut and placed on poly-L-lysine coated slides. The sections were deparaffinized in a stove at 45oC for 30 minutes and subsequently left in xylol for 5 minutes. The sections were hydrated with decreasing concentrations of absolute alcohols (90%, 70% and 50%) and rinsed with distilled water. To unmask epitopes, the antigen retrieval technique was used with a 10mM (pH 6.2) sodium citrate solution using a pressure cooker in microwave oven at full power (750 W) for 5 minutes. Then they were left to cool to room temperature and subsequently rinsed with distilled water. Endogenous Perixodase were blocked with hydrogen peroxide (0.9%), followed by washes with distilled water and phosphate buffered saline pH 7.4 (PBS). Monoclonal primary antibodies were incubated against CK 14 (Clone N3C3 Genetex, CA, USA, dilution 1:100) and against CK 19 (Clone RCK108, Dako Corporation, Carpinteria CA, USA, dilution 1:100) for 45 minutes. The sections were then incubated with the second biotinylated anti-mouse/anti-rabbit antibody and with the streptavidine/peroxidasa complex (LSA-B + Labeledstreptavidin-biotin, DakoCorporation, Carpinteria CA, USA) for 30 minutes each one. The products of the reaction were visualized with 3.3’-diaminobenzidine-H2O2 (DBA) substrate (Dako Corporation, Carpinteria, CA, USA). The sections were counterstained with Mayer’s hematoxylin. Oral mucosa and skin fragments were used as positive controls; as negative controls, the incubation with the primary antibodies was omitted.
Results
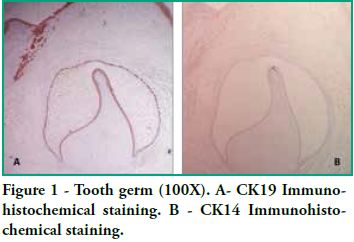
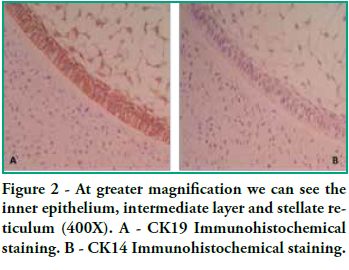
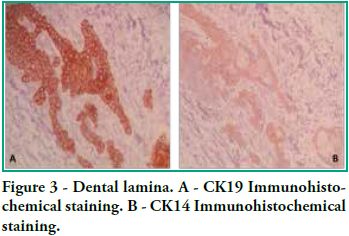
In this study we detected CK14 and CK19 immunoexpression in the epithelium and no expression in the ectomesenchyme both in tooth germs and in ameloblastomas. When observing the enamel organ we can see how CK19 expression varies in the different layers depending on the area observed (Figure 1A). For example, upon analyzing the inner epithelium of the enamel organ, there is stronger CK19 expression on the cell membrane (basal, apical and lateral surfaces); in the intermediate layer the expression was strongly positive for CK19 around the nucleus; the stellate reticulum was strongly positive for CK19 in the extensions, with a lower positive expression in the body cell (Figure 2A), while in the outer epithelium the expression is uniform in the cytoplasm, following the flat shape of the body cell, and finally in the involuting dental lamina we observed intense staining and a homogeneous pattern (Figure 3A). Immunostaining was negative for CK14, except for weak immunostaining observed in the inner epithelium (Figures 1B and 2B). However, there was stronger immunostaining in the dental lamina (Figure 3B).
As for ameloblastomas, three of the six solid/multicystic ameloblastomas included in this study were follicular ameloblastomas and three were plexiform ameloblastomas. All the tumors were positive for CK14 and CK19. Immunopositivity was greater for CK14 than for CK19: there was focal immunostaining for CK19 and mainly diffuse immunostaining for CK14.
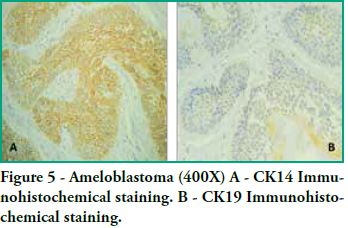
CK19 positivity was predominantly moderate, showing some variations depending on the tumor area, as in follicular ameloblastomas, immunostaining was found in the islands and follicles of odontogenic epithelium with cytoplasmic positivity both in peripheral basal cells with inverse polarization and in central cells that resemble the stellate reticulum. However, said staining was not uniform, since in the tumor itself we found foci that were more positive than others, and inside the same follicles there were areas with positivity variations that did not follow a set pattern (see figure 5). As for plexiform ameloblastomas, plexuses and cords also showed moderate immunostaining which was not uniform, with areas that were more strongly positive than others.
CK14 positivity was in general more intense and less focal, appearing uniformly along all the cords and plexuses of the odontogenic epithelium for the plexiform variation, and for the follicular variety, the follicles and islands of the odontogenic epithelium appeared uniformly in the entire tumor.
Discussion
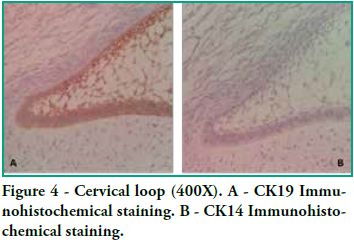
Cytokeratin expression patterns in odontogenic epithelium have been poorly described (10-16). Domingues et al. (10), in their immunohistochemical study, showed that epithelial cells of the tooth germ and the remnants of the dental lamina are positive for CK14 and CK19 with slight changes in their expression pattern, depending on the odontogenesis stage. Our study shows similar results, particularly for CK19, when observing the changes in the inner epithelium from the cervical loop to the future incisal edge, area where the position of calcified material begins. Domingues et al. found that the inner epithelium of the enamel organ was strongly positive for CK14 in the bell stage, while once in the late bell stage, this epithelium, after ameloblast differentiation, showed weaker staining for CK14. This staining appeared in the basal and apical surfaces of the cells. We also found areas of the inner epithelium with weak immunostaining for CK14. In contrast, CK19 showed weak immunostaining in the inner epithelium in the bell stage, but when these cells are differentiated in ameloblasts, the positive intensifies and the cell membrane begins to show (that is to say, the basal, apical and lateral surfaces). Our studies agree with these findings, as we see how the inner epithelium increases CK19 expression as the cells differentiate into ameloblasts. We have also observed that as well as having stronger CK19 staining, its distribution changes. The least differentiated cells present diffuse staining while the most differentiated cells show strong staining in the areas close to the cell membrane (Figure 4). Domingues et al. state that the most prevalent cytokeratin in the remnants of dental lamina was CK14, which strongly stained all cells, while CK19 appeared only in some cells of the dental lamina, with a non-homogeneous pattern. However, in our preparations, staining is strong and has a homogeneous pattern for both cytokeratins (Figure 3). Domingues et al. also observed CK14 expression in the stellate reticulum, expression which was stronger in the early bell stage. They also found CK 19 expression, but it was slightly weaker. In our cases we did not find CK14 immunoexpression, probably due to the fact that they belong to more advanced embryological stages than the early bell stage. As for CK19, we found strong positivity in the extensions, while it was weaker in the body cell (Figure 2). As for the outer epithelium of the enamel organ, they only report staining for CK14 and for CK19; we can only add that CK19 expression is uniform in the cytoplasm, following the flat shape of the body cell.
Crivelini et al. (11), Ferreira Lopes et al. (12), Leon et al. (13), Kasper et al. (14), Heikinheimo et al. (15) and Gao et al. (16) have also studied CK19 expression in tooth germ, although not in depth. They found CK19 in the enamel organ (11-16) and in the dental lamina (11, 13, 14). Crivelini et al. and Leon et al. agree with our findings. They reported that CK14 is gradually replaced by CK19 in the inner epithelium of the enamel organ, with strong CK19 immunoexpression in secretory ameloblasts (15).
Given the changes in the expression of CK14 and CK19 in the inner epithelium of the enamel, some authors have suggested that CK19 could be considered a marker of ameloblast differentiation (10, 11).
Many authors have studied these two cytokeratins in depth in tumors, among which we find odontogenic tumors. They do it to determine their histogenesis or as differential diagnosis for other lesions. Many have shown that the aberrant expression of CK14 and CK19 can be an early finding in the pathogenesis of oral squamous cell carcinoma and can be used as a marker of tumor differentiation (17-19). Several studies show that CK19 expression increases according to the degree and stage of the cancer, including epithelial neoplasms of the oral cavity (20, 21).
Our studies show that CK19 expression was mainly moderate, showing some variations in the positivity depending on the tumor area. As for follicular ameloblastomas, there was immunostaining in the islands and follicles of the odontogenic epithelium. There was cytoplasmic activity both in peripheral basal cells with inverse polarization as in central cells that resemble the stellate reticulum. However, said staining was not uniform, since in the tumor itself we found foci that were more positive than others, and inside the same follicles there were areas with positivity variations that did not follow a set pattern.
Some authors (12, 22, 23) studied CK19 expression in ameloblastomas and report that they found it in all the cells of the tumor, but they do not mention the changes of intensity that we found. Crivelini et al. report finding CK19 expression in some groups of cells with squamous metaplasia and a weak reaction in the central and flat stellate cells of cystic structures, which points to non-uniform staining, as we observed in our studies. They attribute this to the fact that CK19 characterizes only ameloblasts and preameloblasts with complete differentiation, which does not happen in ameloblastomas. In our studies, CK14 positivity was generally more intense and less focal, appearing uniformly along all the cords and plexuses of the odontogenic epithelium for the plexiform variety, and for the follicular variety, the follicles and islands of the odontogenic epithelium were uniformly positive in the entire tumor. Several authors report CK14 positivity in the peripheral cells of the follicles (11, 12, 22), but there is no agreement regarding its presence or not in inner cells: some report negativity in the central area of cells that resemble the stellate reticulum (12), and others say that CK14 is expressed in most inner cells, while, as stated above, we noted CK14 expression in all cells.
We conclude that, as reported by several authors, CK19 can be considered an effective marker of ameloblast differentiation (10, 11, 20, 21) because, when the cells of the inner epithelium differentiate into ameloblasts, immunostaining is usually strongly positive. Conversely, CK14 expression decreases. This points to the participation of these two intermediate filaments in the biology of dental organ formation. Regarding the results observed in ameloblastomas, the fact that the expression of CK14 is stronger than that of CK19 would suggest that these neoplastic cells do not go through ameloblast differentiation. This could be supported by the fact that these tumors do not produce calcified material. Given the inherent limitations of this study, both regarding the number of cases included and the use of only one immunoexpression technique, future studies should be conducted. They should include a representative sample of the different types of ameloblastomas and tooth germs and the use of various experimental approaches.
1. Mass R, Bei M. The genetic control of early tooth development. Crit. Rev. Oral Biol. Med. 1997; 8(1): 4-39.2. Thesleff I, Keränen S, Jernvall J. Enamel knots as signaling centers linking tooth morphogenesis and odontoblast differentiation. Adv. Dent. Res. 2001; 15: 14-18.
3. Oriolo AS, Wald FA, Ramsauer VP, Salas PJ. Intermediate filaments: a role in epithelial polarity. Exp. Cell Res. 2007; 313(10): 2255-2264.
4. Pan X, Hobbs RP, Coulombe PA. The expanding significance of keratin intermediate filaments in normal and diseased epithelia. Curr. Opin. Cell Biol. 2013; 25(1): 47-56.
5. Chung BM, Rotty JD, Coulombe PA. Networking galore: intermediate filaments and cell migration. Curr. Opi. Cell Biol. 2013; 25(5): 600-612.
6. Pallari HM, Eriksson JE. Intermediate filaments as signaling platforms Sci. STKE 2006; (366): 53
7. Kumamoto H. Molecular pathology of odontogenic tumors. J. Oral Pathol. Med. 2006; 35(2): 65-74.
8. Gardner DG, Heikinheimo K, Shear M, Philipsen HP, Coleman H. Odontogenic Tumours. In World Health Organization Classification of Tumors. Pathology and Genetics Head and Neck Tumors. Lyon: IARCPress; 2005. p283-327.
9. Schantz SP. Head and neck oncology research. Curr Opin Oncol. 1994; 6(3): 265-71.
10. Domingues MG, Jaeger MM, Araújo VC, Araújo NS. Expression of cytokeratins in human enamel organ. Eur. J. Oral Sci. 2000; 108(1): 43-47.
11. Crivelini MM, de Araújo VC, de Sousa SO, De Araújo NS. Cytokeratins in epithelia of odontogenic neoplasms. Oral Dis. 2003; 9(1): 1-6.
12. Ferreira Lopes F, Fontoura MC, do Amaral AL, Dantas EJ, Cavalcanti H, Batista L et al. Análise imuno-histoquímica das citoqueratinas em ameloblastoma e tumor odontogênico adenomatóide. J Bras. Patol. Med. Lab. 2005; 41(6): 425-430.
13. Leon JE, Mata GM, Fregnani ER, Carlos-Bregni R, de Almeida OP, Mosqueda-Taylor A et al. Clinicopathological and immunohistochemical study of 39 cases of Adenomatoid Odontogenic Tumour: a multicentric study. Oral Oncol. 2005; 41(8): 835–842.
14. Kasper M, Karsten U, Stosiek P, Moll R. Distribution of intermediate-filament proteins in the human enamel organ: unusually complex pattern of coexpression of cytokeratin polypeptides and vimentin. Differentiation 1989; 40(3): 207-214.
15. Heikinheimo K, Hormia M, Stenman G, Virtanen I, Happonen RP. Patterns of expression of intermediate filaments in ameloblastoma and human fetal tooth germ. J. Oral Pathol. Med. 1989; 18(5): 264-273.
16. Gao Z, Mackenzie IC, Cruchley AT, Williams DM, Leigh I, Lane EB. Cytokeratin expression of the odontogenic epithelia in dental follicles and developmental cysts. J. Oral. Pathol. Med. 1989; 18(2): 63-67.
17. Fillies T, Jogschies M, Kleinheinz J, Brandt B, Joos U, Buerger H. Cytokeratin alteration in oral leukoplakia and oral squamous cell carcinoma. Indian. J. Dent. Res. 2005; 16(1):6-11.
18. Zhong LP, Chen WT, Zhang CP, Zhang ZY. Increased CK19 expression correlated with pathologic differentiation grade and prognosis in oral squamous cell carcinoma patients. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. Endod. 2007; 104(3): 377-84.
19. Ram Prassad VV, Nirmala NR, Kotian MS. Immunohistochemical evaluation of expression of cytokeratin 19 in different histological grades of leukoplakia and oral squamous cell carcinoma. Indian. J. Dent. Res. 2005; 16 (1): 6-11.
20. Babiker AY, Rahmani AH, Abdalaziz MS, Albutti A, Aly SM, Ahmed HG. Expressional analysis of p16 and cytokeratin19 protein in the genesis of oral squamous cell carcinoma patients. Int. J. Clin. Exp. Med. 2014 (6): 1524-30.
21. Schantz SP. Basic science advances in head and neck oncology: the past decade.. Curr Opin Oncol. 1994; 6(3): 265-71.
22. Pal SK, Sakamoto K, Aragaki T, Akashi T, Tamaquachi A. The expression profiles of acidic epithelial keratins in ameloblastoma. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2013; 115(4): 523-531.
23. Fukumashi K, Enokiya Y, Inoue T. Cytoqueratins expression of constituting cells in ameloblastoma. Bull. Tokyo. Dent.. Coll. 2002; 43(1): 13-21
 All the contents of this journal, except where otherwise noted, is licensed under a Creative Commons Attribution License
All the contents of this journal, except where otherwise noted, is licensed under a Creative Commons Attribution License











 text in
text in