Services on Demand
Journal
Article
Related links
Share
Odontoestomatología
On-line version ISSN 1688-9339
Odontoestomatología vol.16 no.24 Montevideo Nov. 2014
Cytokeratins 14 and 19 in odontogenic cysts and tumors: a review
Nieves Sabrina*,
Apellaniz Delmira*,
Tapia Gabriel**
Maglia Alvaro***,
Mosqueda-Taylor Adalberto****,
Bologna-Molina Ronell*****
*Molecular Pathology Trainee, Facultad de Odontología, UdelaR, Uruguay
** Assistant Professor, Grade 3, Department of Histology, Facultad de Odontología, UdelaR, Uruguay
*** Associate Professor, Grade 5, Department of Histology, Facultad de Odontología, UdelaR, Uruguay
**** Specialist in Pathology and Oral Medicine, Full-time Research Professor, Health Care Department, Universidad Autónoma Metropolitana, Mexico
***** Specialist in Pathology and Oral Medicine, Doctor in Biological Sciences (Molecular Pathology), Full-time Associate Professor, Grade 5, Molecular Pathology, Facultad de Odontología, UdelaR, Uruguay. ronellbologna@hotmail.com
Abstract
All mammal cells include a cytoplasmic fiber system essential for cell mobility, the cytoskeleton, formed by three main structural units and associated proteins: microfilaments, microtubules and intermediate filaments. Cytokeratins are intermediate filaments forming a complex network which extends from the nucleus surface to the peripheral cell sector, where they are inserted into desmosomes and hemidesmosomes. Cytokeratins 14 and 19 have been used as diagnosis and prognosis markers in various tumors of epithelial origin, not only to identify a cell as epithelial, but also to identify different stages during epithelial differentiation and to characterize the tumor. There are numerous studies in biomedical literature that have exemplified the utility of cytokeratins 14 and 19 to identify odontogenic epithelium. This review analyzes the utility of their immunologic expression in the different cysts and odontogenic tumors.
Keywords: Cytokeratin 14, Cytokeratin 19, Odontogenic cysts, Odontogenic tumors
Received on: 08 May 2014 – Accepted on: 01 Sep 2014
What are cytokeratins?
All mammal cells include a cytoplasmic fiber system essential for cell mobility: the cytoskeleton. If we try to explain it in a simple and colloquial way, we could compare it to the steel rods that support the structure of a building: the cytoskeleton plays a key role as it supports the plasma membrane and presents paths along which organelles and other cytosol elements can move. However, unlike the passive frame of a building, the cytoskeleton is constantly restructured, which allows for movement (1). The cytoskeleton is formed by three main structural units and associated proteins: microfilaments, microtubules and intermediate filaments. Intermediate filaments are divided into six types according to their molecular characteristics. Cytokeratins (CK) are type I and type II intermediate filaments (2, 3).
Moll et al. (4) classified a total of 19 human epithelial keratins with variable molecular weights within the 40-70 kDa range, and subsequently an additional keratin was identified: CK20. They can be divided into low versus high molecular weight, and into acid or basic according to their isoelectric points (2).
CKs 14 and 19 are type I keratins: CK 19 is the smallest one and it is exceptional because, unlike other cytokeratins, it lacks the typical domain (no alpha helix) (5). CK14 is found in the keratinocytes of stratified squamous epithelium, both in the epidermis and the nonkeratinized mucosa (4), while CK19 is expressed in most simple epithelia, in various ductal epithelia, in intestinal epithelia, in the gastric foveolar epithelium, and in the mesothelium. Besides, it is present in most pseudostratified epithelia and urothelial cells, as well as in basal cells of nonkeratinized stratified squamous epithelium (4).
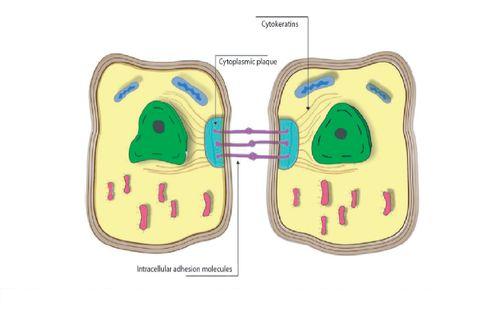
Figure 1: Epithelial cells. Disposition of cytokeratins from the cytoplasmic plaque toward the nuclear periphery.
Which are their functions?
Cytokeratins form a complex network which extends from the nucleus surface to the peripheral cell sector, where they are inserted into desmosomes and hemidesmosomes (Figure 1). As they connect the nucleus surface with the plasma membrane, they provide a permanent link that can have important implications for cytoplasm organization, cell communication, and perhaps for the transportation of information inside and outside the nucleus (2, 4). Additionally, as they are inserted into desmosomes and hemidesmosomes, they contribute not only to the stability of epithelial cells, but to their union with the basal membrane and the underlying connective tissue (4, 6).
In recent years there has been agreement regarding the fact that keratins have two fundamental roles in epithelial cells:
a) structural support, without which physical trauma leads to loss of integrity, and
b) regulation of metabolic processes and their growth, proliferation, migration and apoptosis.
These two general roles involve regulated interactions with a diverse group of
proteins (2, 7), which include signaling molecules such as 14-3-3 proteins, apoptosis-related proteins, kinases and phosphatases (8).
Oriolo et al. (9) say that they can also have a role in epithelial polarity and membrane traffic.
Cytokeratins have also been linked to wound healing, as they provide a more pliable cytoskeleton to the cell, which favors the migration of keratinocytes for wound closure (10).
As for CK14, mutations in the CK genes are responsible for epidermolysis bullosa simplex (11, 12), a hereditary disease, considered important for the physical stability of the epidermis (4). In turn, CKs 15 and 20 have been used as diagnosis and prognosis markers in various tumors of epithelial origin (13, 14).
Utility of cytokeratins as markers
Not all keratins are synthesized simultaneously by a cell, but rather different subsets of keratins are expressed during terminal differentiation in different stages of development, as well as in different epithelia. Therefore, all epithelia (simple and complex) can be classified according to cytokeratin expression. Simple or monostratified epithelia generally express keratins 7, 18, 19 and 20, while complex (stratified) epithelia express keratins 5, 6, 10, 14 and 15. When an epithelium undergoes malignant transformation, its keratin profile usually remains constant (2). Therefore, patterns of keratin expression allow us not only to identify a cell as epithelial, but also to determine the phases of epithelial differentiation and to characterize the tumor. This is why antibodies against several keratins are used routinely at immunohistochemistry labs to diagnose carcinoma, especially unclear metastases (4).
Another clinical application is the detection of protein fragments of CK8, CK18 and CK19 in the bloodstream of cancer patients. These fragments are increasingly used to monitor tumor burden and the progression of the disease in certain carcinomas such as lung cancer (15, 16). Besides, it has been observed that over 95% of lung carcinoma squamous cells are positive for CK14 (13, 17).
CK19 is one of the most frequently studied immunohistochemical markers in thyroid pathology, which could make it a useful diagnosis and prognosis tool, as it can be determined before the therapeutic procedure in cytologic findings (14).
Immunohistochemical staining for CK19 facilitates the differential diagnosis between papillary carcinoma, which presents strong and diffuse staining, and other thyroid cancers, which present weak and focal staining. It has also been suggested that it could be a useful predictor of the progression of thyroid carcinoma (14).
Tsuruba et al. (18) showed that CK8 and CK14 can be useful to diagnose tumors in skin appendices. In general, the following tumors test negative for CK8 and positive for CK14: epidermis, sebaceous glands and hair follicles, while tumors derived from eccrine glands and apocrine sweat glands are CK8 positive and CK14 negative.
Squamous cells carcinoma, as well as malignant mesothelioma, show a strong expression of CK14, while adenocarcinomas show none or very little (2, 4, 19). Studies have suggested that the expression of certain keratins of simple epithelia (like CK19) in squamous cells carcinomas may indicate a poorly differentiated carcinoma (4). CK19 is detected in the epithelium near squamous cells carcinomas, which suggests that it could be used as a fundamental biological agent of the malignant progression (20). Clearly these findings can be applied in the case of oral cavity squamous cells, which shows the utility of these cytokeratins in the diagnosis and as a possible prognosis factor in diverse neoplasms that affect the maxillofacial region (21), as well as lesions of the oral mucosa considered potentially malignant (22, 23).
As for salivary glands tumors, they can be divided into two large categories: tumors derived from stratified epithelium (pleomorphic adenoma, myoepithelioma, basaloid squamous cells carcinoma, adenoid cystic carcinoma and mucoepidermoid carcinoma), and tumors that arise from simple epithelia (adenocarcinoma NOS, monomorphic adenocarcinoma and acinar cell carcinoma). The former express CK14 and CK19, while the latter do not (24, 25). CK14 is a myoepithelial cells marker, therefore, salivary glands tumors with myoepithelial cells are usually positive for CK14. These tumors include benign or malignant myoepithelioma, adenoid cystic carcinoma and pleomorphic adenoma (13, 26, 27).
Cytokeratins and odontogenesis
Patterns of expression of cytokeratins in the odontogenic epithelium have been described in a very general way. Domingues et al. (28), in their immunohistochemical study, showed that epithelial cells of the tooth germ and in the remnants of the dental lamina are positive for CK14 and CK19 with slight changes in their expression pattern, depending on the phase of odontogenesis. For example, they state that at the inner epithelium of the enamel organ the expression of CK14 and CK19 varies in the follicle and bell stages (early bell stage according to the author). They observed a strong positive for CK14 while CK 19 had weak staining, which was the opposite at the late bell or follicle stage. CK19 has a strong positive, while the expression of CK14 decreases. Additionally, the most prevalent cytokeratin in the remnants of the dental lamina was CK14, which strongly stained all cells, while CK19 appeared only in some cells of the dental lamina, with a non-homogeneous pattern. CK14 was also observed in the stellate reticulum, which was stronger at the early bell phase. CK19 was also expressed but more weakly. The outer epithelium of the enamel organ was marked for CK14 and for CK19.
Crivelini et al. (20), Ferreira Lopes et al. (29), Leon et al. (30), Kasper et al. (31), Heikinheimo et al. (32), and Gao et al. (33) also studied the expression of CK14 and CK19 in tooth germ, although in less detail.
They found CK14 and CK19 in the enamel organ (20, 29–33) and in the dental lamina (20, 30, 31).
Crivelini et al. and Leon et al. agree with the findings of Domingues et al. As for CK14, it is gradually replaced by CK19 in the inner epithelium of the enamel organ: immunoexpression of CK19 is very positive in preameloblasts (32).
Given the changes in the expression of CK14 and CK19 in the inner epithelium of the enamel, some researchers have suggested that CK19 could be considered an effective marker of ameloblast differentiation (20, 28).
Odontogenic cysts and tumors
The presence of these cytokeratins in tooth development suggests that they participate in the embryonic development of the dental organ, which is why various authors have studied their expression in odontogenic cysts and tumors (20, 29, 30, 32–47). Patterns of expression of keratins allow us to identify a cell as epithelial and also to identify different stages during epithelial differentiation (4). Antibodies against these cytokeratins have been used to elucidate histogenesis and to characterize the tumor. Numerous biomedical studies have illustrated the utility of these two cytokeratins to identify odontogenic epithelium, and therefore they have been useful in the diagnosis of some neoplasms or cysts where an odontogenic origin is suspected. They have also been useful to determine the possible histogenesis of several cystic or tumoral lesions of the maxillofacial region that are known to be of odontogenic origin (20, 29, 30, 32–34, 37, 40–45, 47). An example of this are the studies conducted in ameloblastomas (20, 29, 34–36), where the expression of CK14 and CK19 was studied to elucidate its possible origin (20, 29). Other authors have studied these same cytokeratins to differentiate them from other lesions, for example Yoon et al., who studied the expression of CK14 and CK19 to differentiate it from ameloblastic carcinoma. They found that in both tumors the expression of both cytokeratins is strong and diffuse. Another interesting study is that of Pal et al. (35), who studied the expression of these same cytokeratins in central ameloblastoma and in peripheral ameloblastoma. They conclude that CK19 expression can be used as a tool to differentiate both neoplasms since in the cases of central ameloblastomas studied, CK19 is positive in all the cells, while in peripheral ameloblastoma, there are CK19 negative cells. Gao et al. (33) studied the expression of CK14 and CK19 in the odontogenic epithelium of normal dental follicles, as well as in the epithelium of dentigerous cysts and keratocystic odontogenic tumors, as they all derive from the odontogenic epithelium of the dental organ. These patterns were compared with cysts of different origins, like nasopalatine cysts and epidermoid cysts. The results obtained confirmed the prediction that development cysts from the odontogenic epithelium shared the expression of CK14 and CK19, but they were different from the cysts originated in non-odontogenic epithelium. Therefore, odontogenic epithelium and its derivates (cysts and tumors) show certain similarities in their patterns of expression of CK, but they differ from nasopalatine cysts for example, which derive from epithelial remnants of the nasopalatine duct, and from epidermoid cysts, which arise from the epithelium during embryonic development.
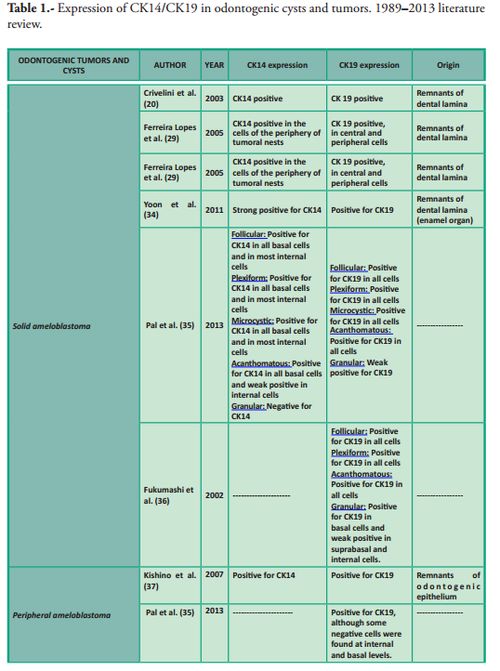
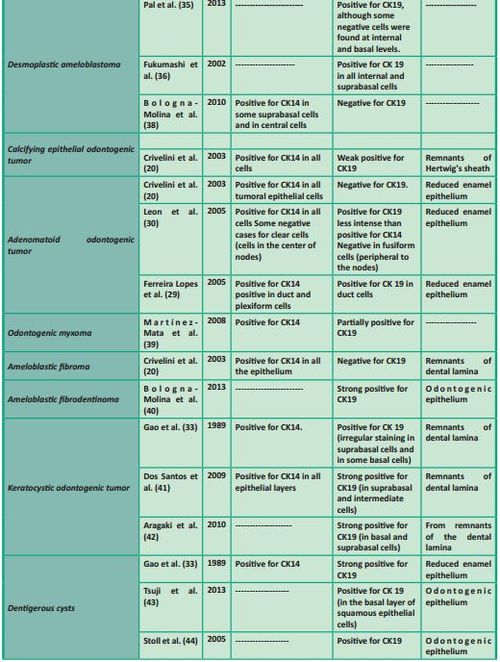
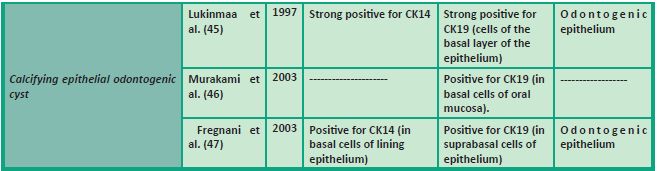
In Table 1 we summarize the studies which have focused on the expression of CK14 and/or CK19 in various odontogenic cysts and tumors.
In conclusion, various studies and recent research increasingly ascribe cytokeratins a role which goes well beyond the mere construction of the cytoskeleton. Several groups are currently studying the influence of their structural and regulatory functions in various diseases, and the molecular interactions of these proteins in various normal and pathological processes are becoming increasingly clear. These new findings highlight the importance of this family of intermediate filaments as useful biomarkers for the prognosis and diagnosis of various human tumors, including those in the oral maxillofacial region.
Nieves Sabrina*,
Apellaniz Delmira*,
Tapia Gabriel**
Maglia Alvaro***,
Mosqueda-Taylor Adalberto****,
Bologna-Molina Ronell*****
*Molecular Pathology Trainee, Facultad de Odontología, UdelaR, Uruguay
** Assistant Professor, Grade 3, Department of Histology, Facultad de Odontología, UdelaR, Uruguay
*** Associate Professor, Grade 5, Department of Histology, Facultad de Odontología, UdelaR, Uruguay
**** Specialist in Pathology and Oral Medicine, Full-time Research Professor, Health Care Department, Universidad Autónoma Metropolitana, Mexico
***** Specialist in Pathology and Oral Medicine, Doctor in Biological Sciences (Molecular Pathology), Full-time Associate Professor, Grade 5, Molecular Pathology, Facultad de Odontología, UdelaR, Uruguay. ronellbologna@hotmail.com
Abstract
All mammal cells include a cytoplasmic fiber system essential for cell mobility, the cytoskeleton, formed by three main structural units and associated proteins: microfilaments, microtubules and intermediate filaments. Cytokeratins are intermediate filaments forming a complex network which extends from the nucleus surface to the peripheral cell sector, where they are inserted into desmosomes and hemidesmosomes. Cytokeratins 14 and 19 have been used as diagnosis and prognosis markers in various tumors of epithelial origin, not only to identify a cell as epithelial, but also to identify different stages during epithelial differentiation and to characterize the tumor. There are numerous studies in biomedical literature that have exemplified the utility of cytokeratins 14 and 19 to identify odontogenic epithelium. This review analyzes the utility of their immunologic expression in the different cysts and odontogenic tumors.
Keywords: Cytokeratin 14, Cytokeratin 19, Odontogenic cysts, Odontogenic tumors
Received on: 08 May 2014 – Accepted on: 01 Sep 2014
What are cytokeratins?
All mammal cells include a cytoplasmic fiber system essential for cell mobility: the cytoskeleton. If we try to explain it in a simple and colloquial way, we could compare it to the steel rods that support the structure of a building: the cytoskeleton plays a key role as it supports the plasma membrane and presents paths along which organelles and other cytosol elements can move. However, unlike the passive frame of a building, the cytoskeleton is constantly restructured, which allows for movement (1). The cytoskeleton is formed by three main structural units and associated proteins: microfilaments, microtubules and intermediate filaments. Intermediate filaments are divided into six types according to their molecular characteristics. Cytokeratins (CK) are type I and type II intermediate filaments (2, 3).
Moll et al. (4) classified a total of 19 human epithelial keratins with variable molecular weights within the 40-70 kDa range, and subsequently an additional keratin was identified: CK20. They can be divided into low versus high molecular weight, and into acid or basic according to their isoelectric points (2).
CKs 14 and 19 are type I keratins: CK 19 is the smallest one and it is exceptional because, unlike other cytokeratins, it lacks the typical domain (no alpha helix) (5). CK14 is found in the keratinocytes of stratified squamous epithelium, both in the epidermis and the nonkeratinized mucosa (4), while CK19 is expressed in most simple epithelia, in various ductal epithelia, in intestinal epithelia, in the gastric foveolar epithelium, and in the mesothelium. Besides, it is present in most pseudostratified epithelia and urothelial cells, as well as in basal cells of nonkeratinized stratified squamous epithelium (4).

Figure 1: Epithelial cells. Disposition of cytokeratins from the cytoplasmic plaque toward the nuclear periphery.
Which are their functions?
Cytokeratins form a complex network which extends from the nucleus surface to the peripheral cell sector, where they are inserted into desmosomes and hemidesmosomes (Figure 1). As they connect the nucleus surface with the plasma membrane, they provide a permanent link that can have important implications for cytoplasm organization, cell communication, and perhaps for the transportation of information inside and outside the nucleus (2, 4). Additionally, as they are inserted into desmosomes and hemidesmosomes, they contribute not only to the stability of epithelial cells, but to their union with the basal membrane and the underlying connective tissue (4, 6).
In recent years there has been agreement regarding the fact that keratins have two fundamental roles in epithelial cells:
a) structural support, without which physical trauma leads to loss of integrity, and
b) regulation of metabolic processes and their growth, proliferation, migration and apoptosis.
These two general roles involve regulated interactions with a diverse group of
proteins (2, 7), which include signaling molecules such as 14-3-3 proteins, apoptosis-related proteins, kinases and phosphatases (8).
Oriolo et al. (9) say that they can also have a role in epithelial polarity and membrane traffic.
Cytokeratins have also been linked to wound healing, as they provide a more pliable cytoskeleton to the cell, which favors the migration of keratinocytes for wound closure (10).
As for CK14, mutations in the CK genes are responsible for epidermolysis bullosa simplex (11, 12), a hereditary disease, considered important for the physical stability of the epidermis (4). In turn, CKs 15 and 20 have been used as diagnosis and prognosis markers in various tumors of epithelial origin (13, 14).
Utility of cytokeratins as markers
Not all keratins are synthesized simultaneously by a cell, but rather different subsets of keratins are expressed during terminal differentiation in different stages of development, as well as in different epithelia. Therefore, all epithelia (simple and complex) can be classified according to cytokeratin expression. Simple or monostratified epithelia generally express keratins 7, 18, 19 and 20, while complex (stratified) epithelia express keratins 5, 6, 10, 14 and 15. When an epithelium undergoes malignant transformation, its keratin profile usually remains constant (2). Therefore, patterns of keratin expression allow us not only to identify a cell as epithelial, but also to determine the phases of epithelial differentiation and to characterize the tumor. This is why antibodies against several keratins are used routinely at immunohistochemistry labs to diagnose carcinoma, especially unclear metastases (4).
Another clinical application is the detection of protein fragments of CK8, CK18 and CK19 in the bloodstream of cancer patients. These fragments are increasingly used to monitor tumor burden and the progression of the disease in certain carcinomas such as lung cancer (15, 16). Besides, it has been observed that over 95% of lung carcinoma squamous cells are positive for CK14 (13, 17).
CK19 is one of the most frequently studied immunohistochemical markers in thyroid pathology, which could make it a useful diagnosis and prognosis tool, as it can be determined before the therapeutic procedure in cytologic findings (14).
Immunohistochemical staining for CK19 facilitates the differential diagnosis between papillary carcinoma, which presents strong and diffuse staining, and other thyroid cancers, which present weak and focal staining. It has also been suggested that it could be a useful predictor of the progression of thyroid carcinoma (14).
Tsuruba et al. (18) showed that CK8 and CK14 can be useful to diagnose tumors in skin appendices. In general, the following tumors test negative for CK8 and positive for CK14: epidermis, sebaceous glands and hair follicles, while tumors derived from eccrine glands and apocrine sweat glands are CK8 positive and CK14 negative.
Squamous cells carcinoma, as well as malignant mesothelioma, show a strong expression of CK14, while adenocarcinomas show none or very little (2, 4, 19). Studies have suggested that the expression of certain keratins of simple epithelia (like CK19) in squamous cells carcinomas may indicate a poorly differentiated carcinoma (4). CK19 is detected in the epithelium near squamous cells carcinomas, which suggests that it could be used as a fundamental biological agent of the malignant progression (20). Clearly these findings can be applied in the case of oral cavity squamous cells, which shows the utility of these cytokeratins in the diagnosis and as a possible prognosis factor in diverse neoplasms that affect the maxillofacial region (21), as well as lesions of the oral mucosa considered potentially malignant (22, 23).
As for salivary glands tumors, they can be divided into two large categories: tumors derived from stratified epithelium (pleomorphic adenoma, myoepithelioma, basaloid squamous cells carcinoma, adenoid cystic carcinoma and mucoepidermoid carcinoma), and tumors that arise from simple epithelia (adenocarcinoma NOS, monomorphic adenocarcinoma and acinar cell carcinoma). The former express CK14 and CK19, while the latter do not (24, 25). CK14 is a myoepithelial cells marker, therefore, salivary glands tumors with myoepithelial cells are usually positive for CK14. These tumors include benign or malignant myoepithelioma, adenoid cystic carcinoma and pleomorphic adenoma (13, 26, 27).
Cytokeratins and odontogenesis
Patterns of expression of cytokeratins in the odontogenic epithelium have been described in a very general way. Domingues et al. (28), in their immunohistochemical study, showed that epithelial cells of the tooth germ and in the remnants of the dental lamina are positive for CK14 and CK19 with slight changes in their expression pattern, depending on the phase of odontogenesis. For example, they state that at the inner epithelium of the enamel organ the expression of CK14 and CK19 varies in the follicle and bell stages (early bell stage according to the author). They observed a strong positive for CK14 while CK 19 had weak staining, which was the opposite at the late bell or follicle stage. CK19 has a strong positive, while the expression of CK14 decreases. Additionally, the most prevalent cytokeratin in the remnants of the dental lamina was CK14, which strongly stained all cells, while CK19 appeared only in some cells of the dental lamina, with a non-homogeneous pattern. CK14 was also observed in the stellate reticulum, which was stronger at the early bell phase. CK19 was also expressed but more weakly. The outer epithelium of the enamel organ was marked for CK14 and for CK19.
Crivelini et al. (20), Ferreira Lopes et al. (29), Leon et al. (30), Kasper et al. (31), Heikinheimo et al. (32), and Gao et al. (33) also studied the expression of CK14 and CK19 in tooth germ, although in less detail.
They found CK14 and CK19 in the enamel organ (20, 29–33) and in the dental lamina (20, 30, 31).
Crivelini et al. and Leon et al. agree with the findings of Domingues et al. As for CK14, it is gradually replaced by CK19 in the inner epithelium of the enamel organ: immunoexpression of CK19 is very positive in preameloblasts (32).
Given the changes in the expression of CK14 and CK19 in the inner epithelium of the enamel, some researchers have suggested that CK19 could be considered an effective marker of ameloblast differentiation (20, 28).
Odontogenic cysts and tumors
The presence of these cytokeratins in tooth development suggests that they participate in the embryonic development of the dental organ, which is why various authors have studied their expression in odontogenic cysts and tumors (20, 29, 30, 32–47). Patterns of expression of keratins allow us to identify a cell as epithelial and also to identify different stages during epithelial differentiation (4). Antibodies against these cytokeratins have been used to elucidate histogenesis and to characterize the tumor. Numerous biomedical studies have illustrated the utility of these two cytokeratins to identify odontogenic epithelium, and therefore they have been useful in the diagnosis of some neoplasms or cysts where an odontogenic origin is suspected. They have also been useful to determine the possible histogenesis of several cystic or tumoral lesions of the maxillofacial region that are known to be of odontogenic origin (20, 29, 30, 32–34, 37, 40–45, 47). An example of this are the studies conducted in ameloblastomas (20, 29, 34–36), where the expression of CK14 and CK19 was studied to elucidate its possible origin (20, 29). Other authors have studied these same cytokeratins to differentiate them from other lesions, for example Yoon et al., who studied the expression of CK14 and CK19 to differentiate it from ameloblastic carcinoma. They found that in both tumors the expression of both cytokeratins is strong and diffuse. Another interesting study is that of Pal et al. (35), who studied the expression of these same cytokeratins in central ameloblastoma and in peripheral ameloblastoma. They conclude that CK19 expression can be used as a tool to differentiate both neoplasms since in the cases of central ameloblastomas studied, CK19 is positive in all the cells, while in peripheral ameloblastoma, there are CK19 negative cells. Gao et al. (33) studied the expression of CK14 and CK19 in the odontogenic epithelium of normal dental follicles, as well as in the epithelium of dentigerous cysts and keratocystic odontogenic tumors, as they all derive from the odontogenic epithelium of the dental organ. These patterns were compared with cysts of different origins, like nasopalatine cysts and epidermoid cysts. The results obtained confirmed the prediction that development cysts from the odontogenic epithelium shared the expression of CK14 and CK19, but they were different from the cysts originated in non-odontogenic epithelium. Therefore, odontogenic epithelium and its derivates (cysts and tumors) show certain similarities in their patterns of expression of CK, but they differ from nasopalatine cysts for example, which derive from epithelial remnants of the nasopalatine duct, and from epidermoid cysts, which arise from the epithelium during embryonic development.
In Table 1 we summarize the studies which have focused on the expression of CK14 and/or CK19 in various odontogenic cysts and tumors.



In conclusion, various studies and recent research increasingly ascribe cytokeratins a role which goes well beyond the mere construction of the cytoskeleton. Several groups are currently studying the influence of their structural and regulatory functions in various diseases, and the molecular interactions of these proteins in various normal and pathological processes are becoming increasingly clear. These new findings highlight the importance of this family of intermediate filaments as useful biomarkers for the prognosis and diagnosis of various human tumors, including those in the oral maxillofacial region.
1. Lodish H, Berk A, Zipursky S, Matsudaira P, Baltimare D, Darnell J. Movilidad y forma de la célula I: microfilamentos. En Biología celular y molecular. 4ed. Buenos Aires: Panamericana, 2002: 751-794.
2. Chu PG, Weiss LM. Keratin expression in human tissues and neoplasms. Histopathology. 2002; 40(5): 403–439.
3. Chung BM, Rotty JD, Coulombe PA. Networking galore: intermediate filaments and cell migration. Curr. Opi. Cell. Biol. 2013; 25(5): 600-612.
4. Moll R, Franke WW, Schiller DL, Geiger B, Krepler R. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell. 1982; 31(1): 11–24.
5. Bader BL, Magin TM, Hatzfeld M, Franke WW. Amino acid sequence and gene organization of cytokeratin no. 19, an exceptional tail-less intermediate filament protein. EMBO J. 1986; 5(8): 1865-1875.
6. Moll R, Franke WW, Volc-Platzer B, Krepler R. Different keratin polypeptides in epidermis and other epithelia of human skin: a specific cytokeratin of molecular weight 46,000 in epithelia of the pilosebaceous tract and basal cell epitheliomas. J. Cell. Biol. 1982; 95(1): 285–295.
7. Pan X, Hobbs RP, Coulombe PA. The expanding significance of keratin intermediate filaments in normal and diseased epithelia. Curr. Opin. Cell. Biol. 2013; 25(1): 47-56.
8. Toivola DM, Strnad P, Habtezion A, Omary MB. Intermediate filaments take the heat as stress proteins. Trends. Cell. Biol. 2010; 20(2): 79-91.
9. Oriolo AS, Wald FA, Ramsauer VP, Salas PJ. Intermediate filaments: a role in epithelial polarity. Exp. Cell. Res. 2007; 313(10): 2255-2264.
10. Magin TM, Vijayaraj P, Leube RE. Structural and regulatory functions of keratins. Exp. Cell. Res. 2007; 313(10): 2021-2032.
11. Omary MB, Coulombe PA, McLean WH. Intermediate filament proteins and their associated diseases. N. Engl. J. Med. 2004; 351(20): 2087–2100.
12. Lane EB, McLean WH. Keratins and skin disorders. J. Pathol. 2004; 204(4): 355–366.
13. Chu PG, Lyda MH, Weiss LM. Cytokeratin 14 expression in epithelial neoplasms: a survey of 435 cases with emphasis on its value in differentiating squamous cell carcinomas from other epithelial tumours. Histopathology. 2001; 39(1): 9–16.
14. Isic Dencic T, Cvejic D, Paunovic I, Tatic S, Havelka M, Savin S. Cytokeratin19 expression discriminates papillary thyroid carcinoma from other thyroid lesions and predicts its aggressive behavior. Med. Oncol. 2013; 30(1): 362.
15. Barak V, Goike H, Panaretakis KW, Einarsson R. Clinical utility of cytokeratins as tumor markers. Clin. Biochem. 2004; 37(7): 529–540.
16. Linder S. Cytokeratin markers come of age. Tumour. Biol. 2007; 28(4): 189–195.
17. Lyda MH, Weiss LM. Immunoreactivity for epithelial and neuroendocrine antibodies are useful in the differential diagnosis of lung carcinomas. Hum. Pathol. 2000; 31(8): 980–987.
18. Tsubura A, Okada H, Sasaki M, Dairkee SH, Morii S. Immunohistochemical demonstration of keratins 8 and 14 in benign tumours of the skin appendage. Virchows Arch. A Pathol. Anat. Histopathol. 1991; 418(6): 503–507.
19. Moll R. Cytokeratins as markers of differentiation in the diagnosis of epithelial tumors. Subcell. Biochem. 1998; 31: 205–262.
20. Crivelini MM, de Araújo VC, de Sousa SO, De Araújo NS. Cytokeratins in epithelia of odontogenic neoplasms. Oral. Dis. 2003; 9(1): 1-6.
21. Fillies T, Jogschies M, Kleinheinz J, Brandt B, Joos U, Buerger H. Cytokeratin alteration in oral leukoplakia and oral squamous cell carcinoma. Oncol. Rep. 2007; 18(3): 639-643.
22. Rivarola de Gutierrez E, Innocenti AC, Cippitelli MJ, Salomón S, Vargas-Roig LM. Determination of cytokeratins 1, 13 and 14 in oral lichen planus. Med. Oral. Patol. Oral. Cir. Bucal. 2014;19(4):e359-65
23. Nanda KD, Ranganathan K, Devi U, Joshua E. Increased expression of CK8 and CK18 in leukoplakia, oral submucous fibrosis, and oral squamous cell carcinoma: an immunohistochemistry study. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. 2012;113(2):245-53 24.
24. Tsubochi H, Suzuki T, Suzuki S, Ohashi Y, Ishibashi S, Moriya T et al. Immunohistochemcial study of basaloid squamous cell carcinoma, adenoid cystic and mucoepidermoid carcinoma in the upper aerodigestive tract. Anticancer Res. 2000; 20(2B): 1205–1211.
25. de Araújo VC, de Sousa SO, Carvalho YR, de Araújo NS. Application of immunohistochemistry to the diagnosis of salivary gland tumors. Appl. Immunohistochem. Mol. Morphol. 2000; 8(3): 195–202.
26. Ogawa Y, Toyosawa S, Ishida T, Ijuhin N. Keratin 14 immunoreactive cells in pleomorphic adenomas and adenoid cystic carcinomas of salivary glands. Virchows Arch. 2000; 437(1): 58–68.
27. Nagao T, Sugano I, Ishida Y, Tajima Y, Matsuzaki O, Konno A et al. Salivary gland malignant myoepithelioma: a clinicopathologic and immunohistochemical study of ten cases. Cancer. 1998; 83(7): 1292–1299.
28. Domingues MG, Jaeger MM, Araújo VC, Araújo NS. Expression of cytokeratins in human enamel organ. Eur. J. Oral. Sci. 2000; 108(1): 43-47.
29. Ferreira Lopes F, Fontoura MC, do Amaral AL, Dantas EJ, Cavalcanti H, Batista L et al. Análise imuno-histoquímica das citoqueratinas em ameloblastoma e tumor odontogênico adenomatóide. J. Bras. Patol. Med. Lab. 2005; 41(6): 425-430.
30. Leon JE, Mata GM, Fregnani ER, Carlos-Bregni R, de Almeida OP, Mosqueda-Taylor A et al. Clinicopathological and immunohistochemical study of 39 cases of Adenomatoid Odontogenic Tumour: a multicentric study. Oral. Oncol. 2005; 41(8): 835–842.
31. Kasper M, Karsten U, Stosiek P, Moll R. Distribution of intermediate-filament proteins in the human enamel organ: unusually complex pattern of coexpression of cytokeratin polypeptides and vimentin. Differentiation 1989; 40(3): 207-214.
32. Heikinheimo K, Hormia M, Stenman G, Virtanen I, Happonen RP. Patterns of expression of intermediate filaments in ameloblastoma and human fetal tooth germ. J. Oral. Pathol. Med. 1989; 18(5): 264-273.
33. Gao Z, Mackenzie IC, Cruchley AT, Williams DM, Leigh I, Lane EB. Cytokeratin expression of the odontogenic epithelia in dental follicles and developmental cysts. J. Oral. Pathol. Med. 1989; 18(2): 63-67.
34. Yoon HJ, Jo BC, Shin WJ, Cho YA, Lee JI, Hong SP et al. Comparative immunohistochemical study of ameloblastoma and ameloblastic carcinoma. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. Endod. 2011; 112(6): 767-776.
35. Pal SK, Sakamoto K, Aragaki T, Akashi T, Tamaquachi A. The expression profiles of acidic epithelial keratins in ameloblastoma. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. 2013; 115(4): 523-531.
36. Fukumashi K, Enokiya Y, Inoue T. Cytoqueratins expression of constituting cells in ameloblastoma. Bull. Tokyo. Dent. Coll. 2002; 43(1): 13-21
37. Kishino M, Murakami S, Yuki M, Lida S, Ogawa Y, Kogo M et al. A immunohistochemical study of the peripheral ameloblastoma. Oral. Dis. 2007; 13(6): 575–580.
38. Bologna-Molina R, Mosqueda-Taylor A, de Almeida-Osley P, Toral-Rizo V, Martínez-Mata G. Peripheral desmoplastic ameloblastoma: histopathological and immunohistochemical profile of a case. Med. Oral. Patol. Oral. Cir. Bucal. 2010; 15 (6): 846-849.
39. Martínez-Mata G, Mosqueda-TaylorA, Carlos-Bregni R, de Almeida OP, Contreras-Vidaurre E, Vargas PA et al. Odontogenic myxoma: clinico-pathological, immunohistochemical and ultrastructural findings of a multicentric series. Oral. Oncol. 2008; 44(6): 601– 607.
40. Bologna-Molina R, Salazar-Rodríguez S, Bedoya-Borella AM, Carreón-Burciaga RG, Tapia-Repetto G, Molina-Frechero N. A histopathological and inmunohistochemical analysis of ameloblastic fibrodentinoma. Case. Rep. Pathol. 2013; 1-7.
41. Dos Santos JN, Oliveira GQ, Gurgel CA, de Souza RO, Sales CB, de Aquiar Pires Valenca Neto A, et al. Altered expression of cytokeratins in primary, recurrent and syndrome keratocystic odontogenic tumors. J. Mol. Histol. 2009; 40(4): 269–275.
42. Aragaki T, Michi Y, Katsube K, Uzawa N, Okada N, Akashi T et al. Comprehensive keratin profiling reveals different histopathogenesis of keratocystic odontogenic tumor and orthokeratinized odontogenic cyst. Hum. Pathol. 2010; 41(12): 1718–1725.
43. Tsuji K, Wato M, Hayashi T, Yasuda N, Matsushita T, Ito T et al. The expression of cytokeratin in keratocystic odontogenic tumor, orthokeratinized odontogenic cyst, dentigerous cyst, radicular cyst and dermoid cyst. Med. Mol. Morphol. 2013.
44. Stoll C, Stollenwerk C, Riediger D, Mittermayer C, Alfer J. Cytokeratin expression patterns for distinction of odontogenic keratocysts from dentigerous and radicular cysts. J. Oral. Pathol. Med. 2005; 34(9): 558-564.
45. Lukinmaa PL, Leppaniemi A, Hietanen J, Allemanni G, Zardi L. Features of odontogenesis and expression of cytokeratins and tenascin-C in three cases of extraosseous and intraosseous calcifying odontogenic cyst. J. Oral. Pathol. Med. 1997; 26(6): 265-272.
46. Murakami S, Koike Y, Matsuzaka K, Ohata H, Uchiyama T, Inoue T. A case of calcifying odontogenic cyst with numerous calcifications: immunohistochemical analysis. Bull. Tokyo. Dent. Coll. 2003; 44(2): 61-66.
47. Fregnani ER, Pires FR, Quezada RD, Shih IeM, Vargas PA, de Almeida OP. Calcifying odontogenic cyst: clinicopathological features and immunohistochemical profile of 10 cases. J. Oral. Pathol. Med. 2003; 32(3): 163-170.











 text in
text in 


