BACKGROUND
Description of liver transplant (LTX) surgery and the intervention
Patients who are candidates for LTX usually present a tendency to bleed but occasionally they might have thrombosis due to the presence of portal hypertension and the deficit in metabolism of factors that lyse the clot. During the LTX, other changes, such as loss of coagulation factors, platelet consumption, heparin-simile phenomena, alter the previous balance, and severe coagulation disorders are common. Conventional coagulation tests are not precise and coagulation monitoring deserves special attention in TLX 1), (2), (3. Thromboelastography (TEG) and thromboelastometry (ROTEM) can be use as "point of care" tests (POC) allowing immediate therapeutic decisions4. The objective of using TEG/ROTEM protocols do guide blood product replacement in LTX is to anticipate to coagulation disorders, avoiding situations that lead to massive indiscriminate transfusions5. TEG/ROTEM discriminate which stage of coagulation is altered, so, the appropriate blood product can be transfused6), (7. The reduction of complications related to transfusions, as well as the reduction of the volume of transfused blood components could reduce the costs of surgery8. Another advantage of TEG/ROTEM is that is the only coagulation monitor or test that can diagnose hypercoagulability disorders, which can be presented in LTX and may easily lead to life threating conditions9.
Background and Importance of this revision
In 2011 TEG/ROTEM could not be recommended for LYX due to the lack of evidence of benefits in outcomes 10. Despite this, the use of TEG/ROTEM has expanded and new clinical studies have emerged 11), (12), (13), (14), (15), (16), (17), (18. The recommendations we made in 2011 need to be update.
The accuracy of the TEG/ROTEM as diagnostic test has been proved19 and systematic reviews were performed to aggregate the evidence from different clinical scenarios (mainly cardiac surgery) 19), (20), (21), (22. A Cochrane systematic review, analysed measures to reduce bleeding in LTX, even though it was focused on pharmacologic treatment, the authors suggested that TEG may be beneficial in reducing blood loss and transfusions 23.
OBJETIVE
Assess the impact of the intraoperative point of care use of TEG or ROTEM versus conventional coagulation tests (CCT) on the blood components transfusion, bleeding, complications, mortality, hospitalization and costs during adult LTX surgeries.
METHODOLOGY
I define the research question and design study as suggested by Carl Counsell in 1997 24. Even if the data aimed to collect are quantitative, I am not willing to statistically analyse the data to perform a meta-analysis due to time limitations.
Criteria to consider studies for this review
I used PICOS framework to establish the research questions (objectives section) and the inclusion criteria(25). Type of studies included. The eligibility criteria were randomized controlled trials and non-randomized controlled trials (RCTs and non-RCTs). The rationale for the inclusion criteria was based on the difficulty of performing a randomization in the context of LTX surgery. The usefulness of TEG/ROTEM was demonstrated in different scenarios; therefore, if a patient has a life-threatening bleeding (which is common in LT), it wouldn’t be ethical to deny the TEG/ROTEM because he/she is assigned to the control group of an RCT. The trials were included irrespective of blinding or sample size. We will limit this study trials with data collected prospectively, regardless of whether the analysis was done prospectively or retrospectively. Cohort study(CS), case-control studies and other non-randomized comparative trials are included.
Exclusions criteria. Trials where: the comparative group didn’t match with the intervention group, the main outcomes are limited to the postoperative period, doesn’t follow TEG/ROTEM guided algorithm or were not written in English, Spanish, Portuguese, French or Italian.
Types of participants
Patients undergoing LTX irrespective of age, donor (living or cadaveric) and all reasons for transplantation.
The following comparisons will be included:
1-Utilization of intraoperative TEG to diagnose coagulation disorders and guide blood product and fluid replacements versus conventional laboratory blood tests .
2-Utilization of intraoperative ROTEM to diagnose coagulation disorders and guide blood product and fluid replacements versus conventional laboratory blood tests.
Types of interventions
Primary outcomes:
- Mortality at maximal follow up.
- Allogeneic transfusion requirements: packaged red cells (PRC), platelets, fresh frozen plasma(FFP), cryoprecipitates)
- Complications (medical adverse event that may be related to the coagulation status).
Secondary outcomes:
Search method for studies identification
An electronic search was done (limited to human subjects, without limit of time) in five databases: The Cochrane Central Register of Controlled Trials, Ovid-MEDLINE, LILACS, Global Health (February 24t-2018) and
The National Library of Medicine (PubMed) (April 6th-2018). We used a search strategy combining Medical
Subject Headings and keywords and synonymous related to the following areas:
1-Intervention: TEG or ROTEM
2-Setting: Liver transplantation.
3-Outcomes: mortality, transfusion, complications, blood loss, hospitalization.
4-Costs.
Synonymous, truncation and wildcards were used. Appendix 1 (A,B,C,D,E). The topics were combined as represented in the diagram of Figure 1. The strategy used in each database is detailed in the Appendix 1.
The trials selected were those that merge either the four circles or those that merge: liver transplant & TEG/ROTEM with either outcomes or costs.
DATA COLLECTION AND ANALYSIS
Selection of the studies
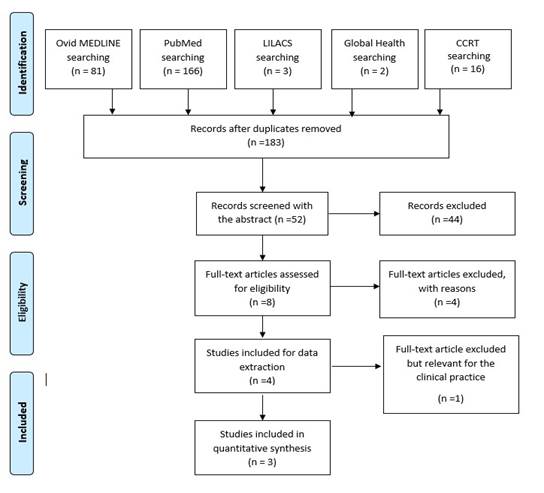
A total of 183 studies were identified and a PRISMA-based diagram was constructed 26 as shown in Appendix 2. We screened the titles and abstracts to identify eligible studies and 8 of them were selected to assess, Appendix 3.
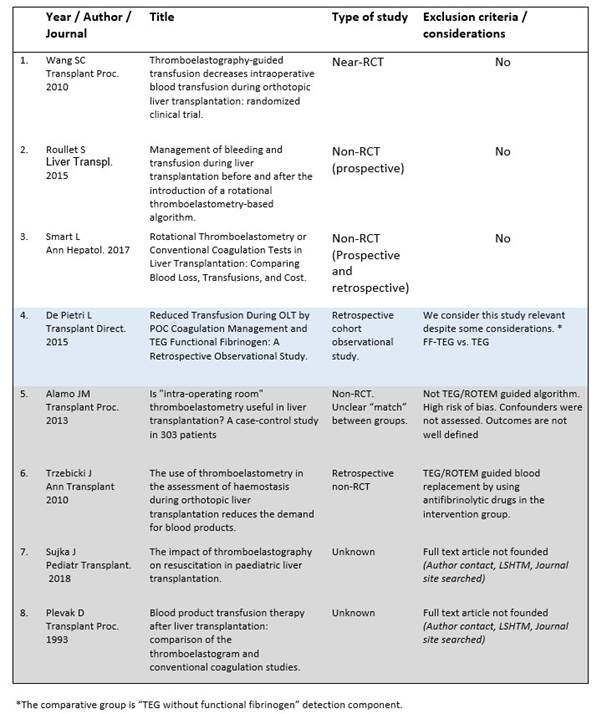
Six of the 8 relevant articles were found in full text and were screened for inclusion and exclusion criteria. A study was excluded because the protocol that TEG/ROTEM protocol was not used to guide fluid replacement but to guide an intermediate pharmacological action: use of antifibrinolytic drugs27. Five trials had the selected outcomes and inclusion criteria and the quality was assessed with a critical appraisal approach to identify bias and confounders 16), (17), (18), (28), (29. Appendix 4 (A, B, C, D, E).
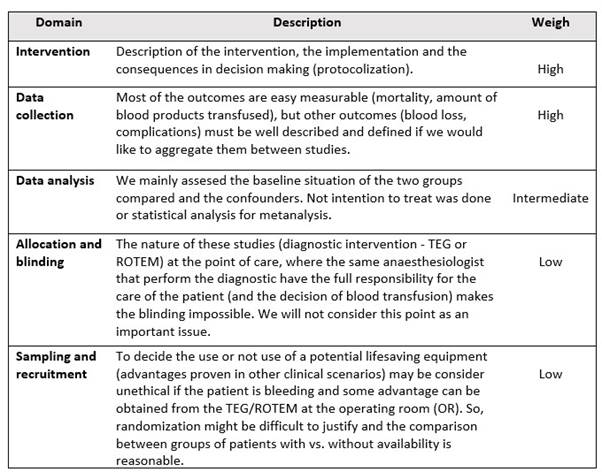
We didn’t define a quality threshold for inclusion criteria because we choose to give up some rigour in favour of the usefulness of this study. The critical quality appraisal was structured in 5 risk domains, and the weigh given to each one depended on the nature of our studied intervention. The “intervention”, “data collection” and “data analysis” domains were considered more important than “allocation and blinding” or “sampling and recruitment”. Appendix 4.
Appraising quality
Internal methodological quality
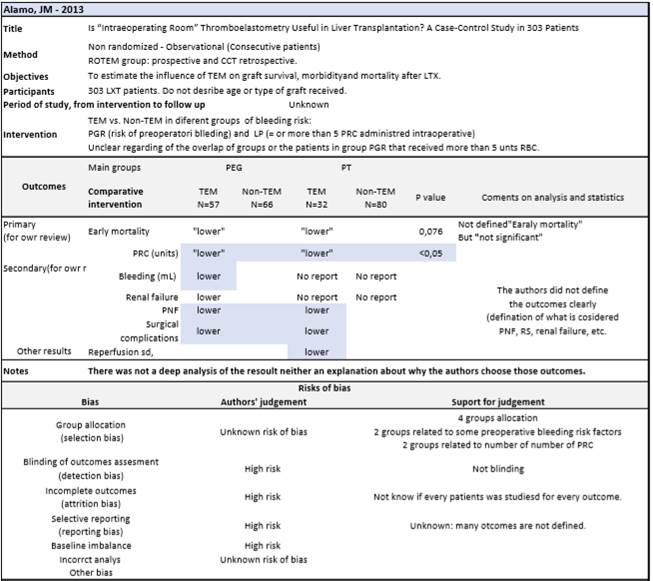
We use the CASP form for the 6 relevant trials and grouped the findings to assess validity of the studies, validity of the results and applicability. Table 1. After discarding the study that had no aimed outcomes 27, 5 studies were assessed for confounders and bias. After the quality appraisal, one article was excluded due to high risk of bias 17. Another study had a control group that was not the target of our study29, even though, we consider that it is relevant for the interpretation of the results. Three included studies had good study validity and applicability. The differences in results will be discussed.
Data collection
The author extracts the data using a modified EPOC worksheets 30. Appendix 5 (A, B ,C ,D ,E). We didn’t perform subgroup analysis because there are few studies and are relatively homogeneous: adult patients and setting.
Bias and confounders of individual studies.
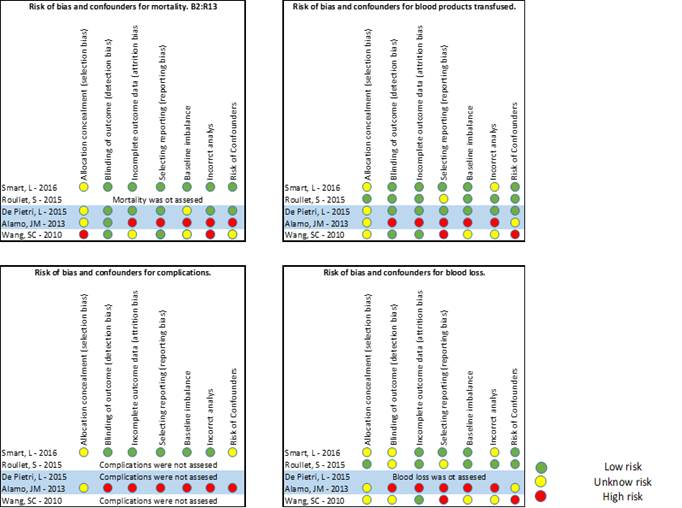
We look for possible confounders and bias from the worksheets and grouped the possible bias (selection, detection, attrition, reporting, baseline imbalance, incorrect analysis) and confounders in 4 diagrams: mortality, blood product replacement, blood loss and deleterious effect of the use of the intervention (Figure 1). Publication bias couldn’t be assessed.
RESULTS
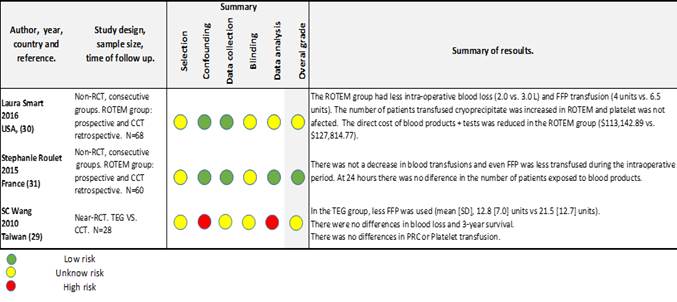
We did a narrative and table synthesis of the outcomes (Table 2).
Results of the search
We found 183 studies that met the searching criteria and 52 were possible relevant. After the abstract screening, we selected 8 studies for full text revision, but only 6 were available 16), (17), (18), (27), (29), (28 (Appendixes 2and3). Three studies were excluded as we explained previously, and 3 studies were analysed in the results.
Risk of bias of individual studies
The result of assessing the bias is presented as a figure with 4 diagrams in that summarises the risk for each group of outcomes studied. Figure 2.
Description of the studies
The 3 studies included 16), (18), (28, reported outcomes from adult LTX patients and were conducted from the intraoperative period. One is a quasi-randomised trial and two are non-RCT well designed with medium to low risk of bias, though we analyse all together and decide not to stratify the results. The sample sizes are 68, 60 and 24 patients. Authors report that TEG/ROTEM protocols were used to guide the blood products replacement, but the algorithm was not explained in Wangs’ study. The control groups had specific triggers from standard laboratory coagulation tests (haemoglobin levels, platelets count, INR value) to replace blood products.
One study 16 included the first 24 postoperative hours for the transfusion results. Smart el all. reported mortality at 60 days and Wang et all. at 3 years, so aggregation was impossible.
Description of the outcomes
Mortality. None of the 2 studies that report mortality (60 days and 3 years) could demonstrate a difference between groups. The number of patients included was very small for the expected mortality in LTX and the risks of bias and confounders for this outcome was considered high to moderate in both studies. Figure-2. Blood products transfused. None of the studies reported a reduction or increase of on PRC or platelets transfusion in the intraoperative period. Roullets’ study showed an increase of the requirements of platelets in first 24 hours.
The transfusion of FFP was reduced in the intervention group in the two studies: Wang, 21.5(SD-12.7) to 12.8(SD-7) unites and Smart, 6.5 (IQR:4-14) to 4(IQR:4-7) units. Roullet et all, report lower amount of intraoperative transfusion of FFP, 8 (IQR:7-8) vs. 4(IQR:4-5), but after 24 hours there was not any difference. The fibrinogen transfusion was studied by Roulette and even it was higher in the intraoperative period in the ROTEM group, the total amount transfused after 24 hours was similar in both groups.
Cryoprecipitates concentrates were studied in Smarts’ study and suggested an increase in the intervention group from 1 to 2 units.
So, we can summarize that with a medium level of evidence, the decrees in blood product transfused in TEG/ROTEM groups are very likely to be limited to FFP, and possibly to the intraoperative period. After 24 hours, the number of patients exposed to blood products may not be affected by the use of TEG/ROTEM.
Blood loss. Smarts’ study (with intermediate risk of bias 18) showed less bleeding in the ROTEM group, but that findings were not confirmed in Roullet or Wang. So, we do not have evidence to say that the TEG/ROTEM directed therapy influences blood loss in adult LTX surgery. The main risk of measurement bias arises from difficulty of estimate intraoperative blood loss and the lack of blindness.
Complications and adverse events. None of the studies assessed the possibilities of complications and morbidity. Smarts’ study compares hospitalization and ICU length of stay and couldn’t demonstrate any differences between groups.
Costs. The costs were only analysed in one study 18 and the data are considered not to be generalizable. Table 2.
DISCUSSION
We use a systematic approach to analyse the findings of 3 studies: one quasi-randomized and two non-RC. The inclusion of non-RCT increases the risks of potential biases (specially selection). In this study concerns arise with respect to differences between groups (selection bias) and from studies that do not explicitly explain the transfusion protocol used (reporting bias).
Altogether, the analyses, suggest a benefit of using a TEG/ ROTEM-guided transfusion therapy to reduce the transfusion of FFP in adult LTX. The reduction in other blood products transfused are not proven in these studies. Better designed studies with more number of patients are needed to assess the benefits of TEG/ROTEM in bleeding.
Our results differ from other systematic reviews conducted recently by Wikkelsø 31 that concluded that TEG/ROTED guided transfusion may reduce the need for blood products in patients with bleeding. The differences may be due to the different setting (mainly cardiac surgery) or the few number of studies and patients included in this review.
De Pietri compared the use of fibrinogen functional TEG (FF-TEG) vs. ROTEM 29) on resource consumption in 386 LTX patients. They concluded FF-TEG guided therapy reduces all blood products used (FFP, PRC and platelets) and an increase of fibrinogen use. This study might be a consideration when performing new studies about the utility of TEG.
The influence in mortality could not be demonstrated. The LTX surgery is extremely complex and it is likely that a lot of confounders make it difficult to assess mortality in this study with a small number of patients included.
In conclusion, TEG/ROTEM directed blood products replacement in LTX might be effective in reducing FFP transfusion during the intraoperative. Further studies are required to confirm this finding and to assess the overall requirements of other blood products, bleeding mortality and complications.
REVIEWS’ limitations and reflections
1-It is desirable that at least two authors to work on a systematic review, but this was an individual assignment.
2-Given the retrospective nature of the reviews, it would have been important to publish or register a formal protocol previously to perform the study (as done in the Cochrane reviews) to reduces biases. If there are later changes in the inclusion criteria, they must be duly justified(25).
3-We should have hand-search the reference list from identified relevant studies and contact the manufacturers of TEG and ROTEM for unpublished trials. If I would have need to contact Sujka, J or would purchase the paper for assess inclusion criteria (32). I should have search for ongoing clinical trials on academic internet sites: ISRCTN registry, Clinical trials registry, Center Watch, UMIN clinical trials registry.
4-Global Health database might not be a relevant source for clinical trials, so I should probably search in other clinical one.
5-I would have performed statistic techniques to integrate the results of included studies due to the quantitative nature of the studies. A meta-analysis could have been done. For dichotomous data with binary outcomes we can calculate the risk ratios (RRs) with 95% CI and for continuous data the standardized mean difference. When the distribution is asymmetric the median value might be used.
6-I would have done an abstract and a plain language summary to making the information more understandable and open to researchers and non-researchers. Nevertheless, we have a strict word limitation and I considered that a better description of methods and results would be more adequate for a Course Assignment, than extending the work with the abstracts.