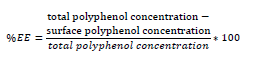1. Introduction
Wine is one of the most consumed alcoholic beverages worldwide. Consequently the wine industry produces large amounts of byproducts1. The main byproduct of winemaking process is grape pomace, which consists mainly of peels and seeds that remain after pressing the grapes2. These wastes from the winemaking process pose a great environmental problem, mainly due to their high organic load that hinders their rapid degradation3, in addition to their seasonal nature, since large quantities are produced during a short period4.
Apart from the wine industry, the cheese industry also generates significant amounts of waste, whey being the most abundant. Whey proteins have numerous bioactive properties, which can be promoted by the release of bioactive peptides encrypted in the native proteins through enzymatic hydrolysis5. In this regard, a great interest has grown in recent years about the use of these agro-food residues, not only due to the environmental problems they generate, but also because of having various health-promoting properties6.
Grape pomace contains 70% of the polyphenols present in the grape1, which are distributed in the seeds and skins. Among the phenolic compounds, anthocyanins are the main ones found in the peel, which have high antioxidant capacity, among other properties7 involved in the prevention of cardiovascular diseases, obesity and diabetes control8)(9. In particular, an ethanolic extract from Tannat grape skin has shown antioxidant, antidiabetic, anti-obesity and anti-inflammatory properties9.
Because of being good antioxidants, anthocyanins have the ability to combat oxidative stress, among other bioactive properties10, which is a risk factor for the development of non-communicable chronic diseases11)(12. According to the World Health Organization (WHO), these diseases kill 41 million people each year, so it is of great interest the search for new components that promote the reduction of oxidative stress13.
In this sense, anthocyanins have shown important bioactive properties such as the ability to inhibit the formation of reactive species9, as well as presenting great potential as a functional ingredient for the development of functional foods. Not only extracts rich in anthocyanins provide bioactive properties, they are also used to replace the use of artificial colorants in different products14)(15, such as yogurt.
Yogurt is a widely consumed functional food which beneficial effects on health have been related to probiotics and the presence of bioactive peptides16. However, yogurt is not considered a source of phenolic compounds, so the addition of extracts rich in polyphenols could be a suitable strategy to increase its content. Phenolic compounds are unstable ingredients against various factors, so their addition to different types of foods represents great challenges17, particularly anthocyanins18. One way to protect bioactive compounds from losing bioactivity during the food shelf life, either through chemical degradation or interaction with other ingredients, is through encapsulation19)(20. Micro-encapsulation technology using correct encapsulating agents could solve the instability drawbacks, alleviate unpleasant tastes, color changes, as well as improve the bioavailability and half-life of the compound20)(21)(22. Whey protein has been shown to be a good bioactive compound encapsulating agent20 as well as inulin, since it has been efficient for the encapsulation of bioactive compounds present in purple cactus pear23. Spray drying is one of the most widely used micro-encapsulation techniques in the food industry, since the equipment allows drying of the microparticles in short times, reducing the loss of activity4)(17)(19)(22.
In the present work, to develop a potential functional yogurt with the addition of bioactive compounds from Tannat grape skin is proposed (byproduct of wine industry), obtained by ethanolic extraction (mainly anthocyanins) and encapsulated by spray drying, which could be also used as a natural colorant.
2. Materials and methods
2.1 Materials and reagents
Tannat grape pomace was provided by Bouza SA wine cellar (Montevideo, Uruguay). Whey protein isolate (Lacprodan® 80) and Orafti®HIS inulin derived from chicory were purchased from Arla Foods Ingredients (Argentina) and Beneo (Belgium), respectively.
Buffer solution salts were Na2HPO4 and KH2PO4 (Sigma-Aldrich, St. Louis, MO, USA). All reagents used in physic-chemical analysis were of reagent grade. Alcalase (P4860 Alcalase® 2.4 L, Bacillus licheniformis, Subtilisin A) was purchased from Novozyme Corp. Dimethyl sulfoxide (DMSO), Folin reagent, 2,2′-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS), 6-hydroxy-2,5,7,8-tetramethylch-roman- 2-acid (Trolox), fluorescein (FL) disodium salt and 2,2′-azo-bis (2-methylpropionamidine) dihydrochloride (AAPH) and CH3COONa.3H2O were from Sigma-Aldrich (St. Louis, MO, USA). Potassium persulphate was purchased from J. T. Baker (New Jersey, USA).
2.2 Treatment and preparation of Tannat grape skin extract
The separation of grape pomace into seeds and skin was carried out manually. The seeds were discarded while the skin was dried at 40°C in a conventional oven until constant weight (24 hours). Subsequently, dried skin was milled using a domestic coffee grinder until obtaining a homogeneous powder.
The ethanolic extraction of the bioactive compounds from grape skin powder was carried out as described by Fernández-Fernández and others1. Briefly, 100 g of powder were weighed and 1 L of 95% ethanol was added. The mixture was left to macerate for 24 hours at room temperature in the dark. Then, the extract was filtered using filter paper (Whatman n°4). For total solvent elimination, the supernatant was subjected to rotary evaporation (BHI (R-114), BUCHI, USA), under reduced pressure, followed by drying in a conventional oven at 40°C for 24 hours (E s/t). E s/t was composed of carbohydrates (15.01 ± 1.34 g/100 g of dry sample) and hydro-soluble protein (0.43 ± 0.05 g/100 g of dry sample)1.
2.3 Preparation of microcapsules
For the encapsulation of the ethanolic extract bioactive compounds, two systems of encapsulating agents were used. The first system consisted of non-hydrolyzed whey protein (WPI) and inulin (I) in a 3:1 ratio according to Agnetti and others24, and the second one of hydrolyzed whey protein (WPIH) and inulin also in a 3:1 ratio. Both systems were prepared in 300 mL of 100 mM phosphate buffer pH 7.0 by weighing 24 g of WPI / WPIH, 8 g of inulin, and for the system corresponding to the hydrolyzed whey protein (WPIH), 2.4 g of enzyme Alcalase was added. Enzymatic hydrolysis was carried out at 30°C in a water bath with constant stirring for 1 h, and the reaction was stopped by heating at 100°C for 10 min.
The encapsulations of the dry extract were carried out by placing 32 g of dry extract with 300 mL of each encapsulating system (1:1 ratio).
The 2 encapsulated systems (WPI+I+Extract; WPIH+I+Extract), and the 2 blank systems (WPI+I; WPIH+I) were subjected to spray drying using a tabletop spray dryer EDIBON SSPB (Leganés, Madrid, Spain), obtaining powder samples. The product feed rate was 3 mL/min, the atomization pressure was 0.14 MPa, the air inlet temperature was 180°C, and the feed temperature was 60°C. These working conditions were selected according to previous work from our group24. The blank solution corresponding to the non-encapsulated extract could not be subjected to spray drying because the anthocyanins precipitated in the phosphate buffer, preventing their passage through the equipment. The non-encapsulated extract was heated in a convection oven for 5 minutes using the same air temperature (180°C; E 180).
All the spray-dried powders were stored at -20°C for subsequent analysis.
2.4 Yogurt preparation
Seven yogurt formulations (Y) were prepared (Figure 1): two yogurts with the addition of encapsulating agent systems (Y WPI+I; Y WPIH+I), two yogurts with the extract encapsulated with the different encapsulating agent systems (Y WPI+I+E; Y WPIH+I+E), a yogurt with the incorporation of the extract subjected to the spray drying temperature (Y E 180), one with the extract without being subjected to temperature (Y E s/t), and a base formulation without the addition of any component (Y B).
Each bottle with 100 g of mix (UHT whole fluid milk and skim powder, cassava starch, unflavored gelatin, inulin and stevia) was inoculated with 0.25 mL of YO-MIX 495 LYO ferment (250 DCU, containing Streptococcus thermophilus and Lactobacillus delbrueckii subspecies bulgaricus). The mixtures corresponding to the yogurts only with extract and only with encapsulating agent were added in a concentration of 0.5% of powder in yogurt, while the other yogurts were added in a concentration of 1 % w/w, to maintain the extract addition ratio in the yogurt formulation (encapsulant:extract 1:1). The mixtures were incubated in the oven at 42°C until reaching a pH of 4.5. Once pH 4.5 was reached, the bottles were placed in a cold-water bath and shaken to obtain a smoothie yogurt, then they were stored at 4°C for further analysis.
2.5 Evaluation of encapsulation efficiency
The systems encapsulation efficiency to encapsulate bioactive compounds was determined by the Folin-Ciocalteau method (described in Section 2.6), by determining the polyphenol content in the whole sample and in the surface of the microcapsules. The content of phenolic compounds on the surface of the nano-microcapsules and the content of free phenolic compounds after destabilization and rupture of the nano-microcapsules were determined.
The content determination of polyphenols on the surface was carried out on the filtrate of the dispersion of 150 mg of nano-microcapsules in 1.5 mL of ethanol:methanol (1:1 v/v) after stirring23.
For the destabilization and rupture of the nano-microparticles, and subsequent determination of the total polyphenol content, 150 mg of nano-microparticles were dispersed in 0.75 mL of acetonitrile and 0.75 mL of methanol:water:acetic acid (50:8:42 v/v/v). It was stirred for 1 minute, centrifuged and filtered23.
The efficiency percentage (EE) was determined by the following equation:
2.6 Determination of the content of total polyphenols
Total polyphenol content (TPC) was determined in the powder and yogurt samples diluted in distilled water using Folin-Ciocalteau method, with the modifications described by Fernández-Fernández and others1. Briefly, 10 µL of standard/sample and 200 µL of 20% sodium carbonate solution were added to the 96-well plate. After 2 minutes, 50 µL of Folin-Ciocalteau reagent (1/5) were added. After 30 minutes in the dark, absorbance was measured at 750 nm in a microplate reader Thermo Scientific Multiskan FC model (Massachusetts, USA). The method was performed using a gallic acid calibration curve (0.05 - 1.0 mg/mL). The results obtained were expressed as mg of gallic acid equivalents (GAE)/100 g of dry sample.
This technique was applied for the determination of total polyphenol content in the surface of the nano-microcapsules and after destabilization and rupture of the nano-microcapsules, as well as in the nano-microcapsules and in the yogurt samples suspended in distilled water.
2.7 Determination of antioxidant capacity
To carry out ABTS and ORAC-FL techniques, the steps described by Fernández-Fernández and others1 were followed. The calibration curve was performed with Trolox at concentrations of 0.25-1.5 mM for ABTS and 0.01-0.08 mM for ORAC-FL. In both cases, the results were expressed as μmol of Trolox Equivalents (TE)/g of sample.
2.8 Determination of color
To determine the color in yogurt formulations, the CIEL*ab color space system was used. The values L, a and b were obtained, being: L*=luminosity, a=tendency of the color to red (positive) or green (negative), b=tendency of the color to yellow (positive) or blue (negative)25.
2.9 Statistical analysis
The analysis of the results was performed by analysis of variance (ANOVA) using Infostat program26. Significant differences were determined using the Tukey test (p<0.05). All the aforementioned analyzes were carried out at least in triplicate.
3. Results and discussion
3.1 Evaluation of encapsulation efficiency
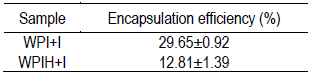
Non-hydrolyzed whey protein and inulin (WPI+I) system presented the highest efficiency to encapsulate the compounds present in the extract (Table 1). Although it is known that proteins are capable of forming complexes with polyphenols, it has been observed that after enzymatic hydrolysis of milk proteins, a weaker or different interaction is generated with polyphenols27 that could affect the encapsulation efficiency20. This is in accordance with the results obtained in the present work, where it was observed that whey protein hydrolysis generated a decrease in the interaction with the polyphenols present in the extract, probably because of the decrease in surface hydrophobicity and interference with the formation of the interfacial layer28.The interaction decrease could have caused less encapsulation by the protein and therefore a lower encapsulation efficiency compared to the system that contains the protein in its native form. The encapsulation efficiency obtained is in agreement with that reported by Hu and others20 for the encapsulation of an ethanolic extract rich in polyphenols by means of nanoparticles of whey protein concentrate. In addition, the encapsulation of grape peel anthocyanins by spray drying has been reported29, as well as other matrices rich in anthocyanins30)(31.
3.2 Determination of total polyphenol content in powder samples
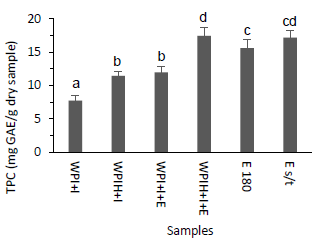
Total polyphenol content was determined in the powder samples (Figure 2). As can be seen, there were no great differences in TPC values between the extract subjected to temperature (E 180) and the extract without temperature (E s/t). This may suggest the temperature of the air at the spray dryer (180°C) would not seem to greatly affect the content of the polyphenols present in the extract in the time of exposure (residence time in the order of seconds)32. Moreover, TPC increased after the addition of the extract in both encapsulating systems, which is why it can be confirmed that the extract obtained from the winemaking byproduct represents a contribution in the content of polyphenols.
It should be noted that the reagent used is not specific for polyphenols since the phenolic groups found in proteins or other substances capable of reducing Folin reagent can also participate in the reduction reaction.
The presence of TPC values in systems without the addition of phenolic compounds (WPI+I and WPIH+I) is due to the fact that the -OH groups present in the proteins that are part of the encapsulating systems are capable of reducing the Folin reagent33. Likewise, it is proposed as a good method to compare total polyphenol content of different samples due to the speed of the test.
3.3 Determination of antioxidant capacity in powder samples
In Figure 3, the results of the antioxidant capacity measured by ORAC-FL (Figure 3a) and ABTS (Figure 3b) of the different powder samples are shown. The same trend was seen in both assays, showing that systems containing hydrolyzed whey protein (WPIH), both with extract and without extract, have greater antioxidant capacity compared to systems containing non-hydrolyzed protein (WPI). The highest antioxidant capacity in systems with protein hydrolysate was due to the release of bioactive peptides with antioxidant capacity, that are encrypted in the native protein, during the hydrolysis of the protein34)(35.
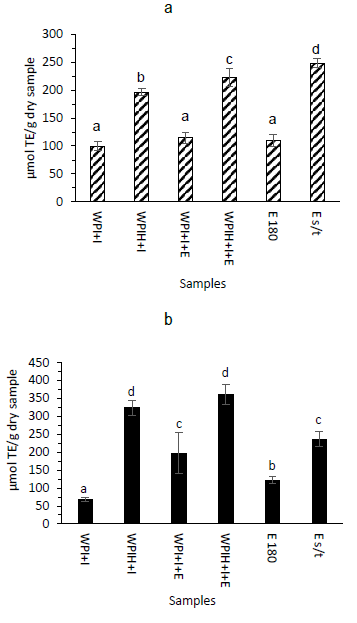
Figure 3: Antioxidant capacity measured by ORAC-FL (a) and ABTS (b) of powder samples on dry basis. Bars denote the mean values and error bars the standard deviation. Different letters represent significant differences according to Tukey test (p < 0.05)
Regarding the antioxidant capacity of the systems only with extract, the one that was not subjected to the temperature of the spray dryer (E s/t) presented a higher antioxidant capacity than the extract subjected to 180°C (E 180). These results suggest that the spray drying temperature affects the polyphenols present in the sample and therefore its antioxidant capacity36. The results of the present work agree with that reported by Kuck and others29 for micro-encapsulated anthocyanins by spray drying. Other authors have reported the dependence of the encapsulation efficiency of anthocyanin rich matrices with different encapsulating agents, increasing the efficiency with the encapsulant composition based on polysaccharides and proteins31.
In the work carried out by Yin and others27 it was shown that the interaction between polyphenols and proteins affects the biological function of polyphenols. Therefore, perhaps the lack of a drastic increase in antioxidant capacity when extract was added to the systems may be due to the fact that the interaction with the encapsulating protein affected the biological function of the anthocyanins present in the extract. In addition, the lack of increase could also be due to the loss of polyphenols as a consequence of the exposure to the working temperatures of the spray dryer because of the low encapsulation efficiency23. In this sense, Lauro and others30 used lower temperatures for drying micro-encapsulated anthocyanins to avoid their degradation, with the negative consequence of obtaining a final product with higher moisture content. The results agree with previous reports on the encapsulation by spray drying of other matrices rich in polyphenols20)(29)(37.
3.4 Determination of antioxidant capacity in yogurts
The highest antioxidant capacities measured by both ORAC-FL and ABTS were observed for the yogurt formulation containing hydrolyzed whey protein (Y WPIH+I and Y WPIH+I+E) (Figure 4), which agrees with the release of antioxidant peptides due to enzymatic hydrolysis5)(38. Moreover, antioxidant capacity was increased by the addition of extract in the case of the ORAC-FL determination (Figure 4a). These results are in accordance with that reported by Iriondo-DeHond and others15, where yogurts with red grape byproduct extracts showed a significant increase in their antioxidant capacity, compared to base formulation of the yogurts.
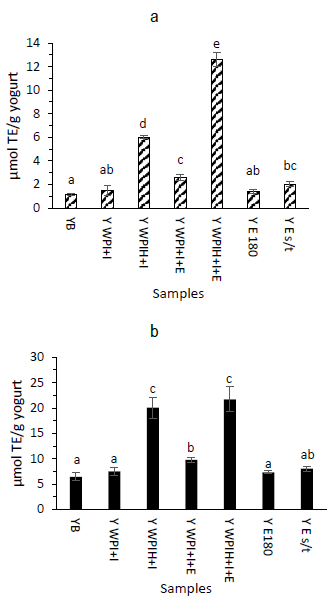
Figure 4: Antioxidant capacity of yogurt formulations by ORAC-FL (a) and ABTS (b). Bars denote the mean values and error bars the standard deviation. Different letters represent significant differences according to Tukey test (p < 0.05)
Yogurt with non-hydrolyzed whey protein encapsulant and extract (Y WPI+I+E) presented higher antioxidant capacity than base formulation (Y B) and extract with heat treatment (Y E180), suggesting a protective effect from the encapsulant regarding anthocyanins degradation. Although the antioxidant capacity of the yogurt with hydrolyzed whey protein (Y WPIH+I) increased, the technological process should be further studied to evaluate its incorporation, since the yogurt with WPIH+I was destabilized under the used conditions observing phase separation. Moreover, the bioactive compounds in Y WPI+I+E were better encapsulated, being less accessible for the determination of antioxidant capacity that could have been released during digestion process, exerting their antioxidant effect. Further studies regarding bioaccessibility should be addressed in order to evaluate the antioxidants after digestion.
3.5 Determination of color in yogurt
As part of the characterization of the developed product, the color results were obtained by the CIEL*ab system (Table 2).
Table 2: Values obtained by colorimeter of the parameters L, a and b of the different yogurt samples
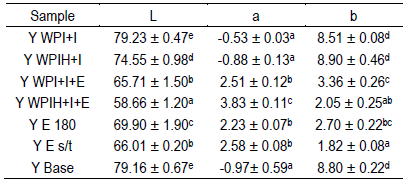
*Results are expressed as mean values ± standard deviation. Different letters show significant differences by Tukey test (p < 0.05).
Regarding luminosity (L), the samples with extract presented lower luminosity values than the samples without extract. This is due to the presence of anthocyanins in the extract, which provide dark coloration to the yogurt, showing greater absorption of light and therefore less luminosity. The lowest value of L obtained (p < 0.05) corresponded to Y WPIH+I+E yogurt, which is consistent with lower encapsulation efficiency than Y WPI+I+E. Yogurt with the incorporation of extract with heat treatment (Y E 180) showed a higher L value (p<0.05) than the previous ones, which could be due to the low homogeneity of the extract in the yogurt and a decrease in the anthocyanin content with respect to the extract without temperature (Y E s/t), as a consequence of heat treatment.
Regarding the “a” value, yogurts with the incorporation of the extract showed positive values, which is consistent with the trend towards the violet color of the yogurt. In turn, between the Y WPIH+I+E and the Y WPI+I+E formulations, the sample with the highest value of "a" (p<0.05), and therefore the highest violet color, was the one that contained hydrolyzed protein (WPIH), showing that the anthocyanins were not encapsulated in the same way as they did in the system with non-hydrolyzed protein.
As to parameter "b", large differences were seen between the samples with and without the incorporation of extract. The formulations of the control yogurts (Y WPI+I, Y WPIH+I and Y Base formulation) presented positive and higher values without significant differences between them (p>0.05), indicating a tendency towards more yellow while the yogurts with the presence of both encapsulated and non-encapsulated extract presented lower “b” values (Y E s/t presented the lowest value without significant differences with Y WPIH+I+E, p>0.05), indicating that they present a color closer to blue.
The incorporation of the extract into the yogurt gave it a purple color, which could be attractive to the consumer. In addition, today there is a growing trend in the use of natural colorants by the industry15 due to the demands of today’s consumers8. The use of micro-encapsulated polyphenols by spray drying from other food matrices as natural colorants in yogurts has been reported4, as well as rich anthocyanin matrices as natural colorants in the formulations of other foods31)(39.
In this sense, the byproduct of wine industry could be used as a natural colorant, contributing to the management of this abundant residue that generates environmental problems.
4. Conclusions
In the present work, encapsulates of natural antioxidants from a Tannat grape winemaking byproduct extract (pomace skin) were developed by spray drying, which could be incorporated into different yogurt formulations. The addition of the encapsulated extract to the yogurt increased the antioxidant capacity with respect to the yogurt base formulation (p<0.05), presenting potential as a functional food. In addition, the formulations of the yogurts with extract addition showed a characteristic violet/purple color that could be attractive to the consumers, thus proposing the extract as a natural colorant to be used for the development of novel yogurts. Future studies should be carried out regarding other bioactive properties of yogurts with the encapsulated extract that may contribute to yogurt health-promoting properties, as well as sensory analysis with consumers to evaluate the acceptability of the novel yogurts.